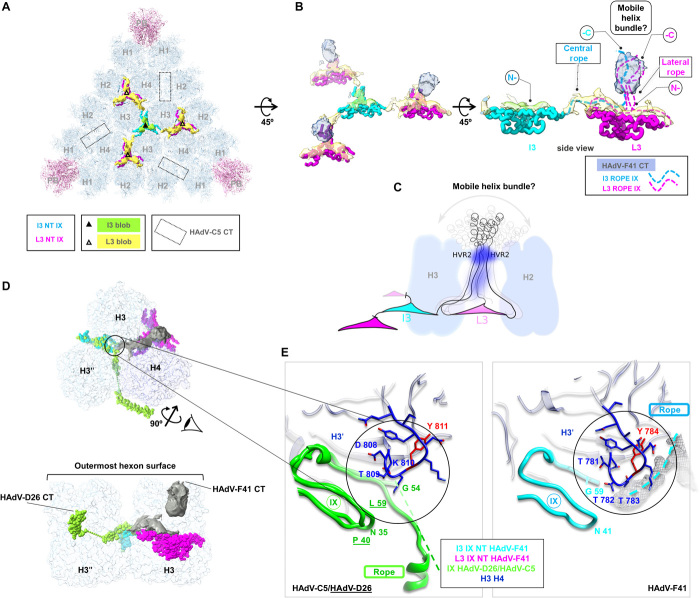
Fig. 4. Organization of protein IX.
(A) External remnant density (green and yellow, unsharpened, 0.2σ). Modelled triskelions are in cyan (I3 axis, I3 NT) and magenta (L3 axes, L3 NT). Dotted rectangles: location of helix bundles in HAdV-C5. (B) Interpretation of the remnant map. Apart from elements shown in (A), density protruding at the L3 axes is in blue, and the proposed path for the rope domains corresponding to each triskelion in dashed lines. (C) Cartoon representing the hypothesis that the C-terminal domains associate forming a mobile, spike-like structure whose density is averaged away. Hexon HVR2 loops would constrain the flexibility of IX, producing the most evident blurry density (dark blue splotch). Less intense dark blue represents weaker density observed in some localized reconstruction classes (fig. S5B). (D) The rope domain of the IX molecules forming the I3 triskelion follows different paths in HAdV-C5/D26 and HAdV-F41. One of the IX monomers forming the HAdV-D26 I3 triskelion is in green; the equivalent monomer in HAdV-F41 in cyan; and the HAdV-F41 L3 triskelion in magenta. Grey surface: HAdV-F41 remnant map. Hexons (H3, H3′, H4) in semi-transparent surface. Top: view from outside the capsid as in (A). Bottom: side view after rotating as indicated. Notice that the blurry density at L3 protrudes above the hexon towers. (E) Zoom in on the region where the triskelion ends and the rope domain turns. Green, overlapped structures of IX in HAdV-C5 and HAdV-D26. HAdV-D26 residue labels are underlined. Dashed lines: untraced rope domains in HAdV-C5 and HAdV-F41. Grey mesh: HAdV-F41 remnant density corresponding to the rope domain. Hexon amino acids with RMSD above 2 Å are in red.