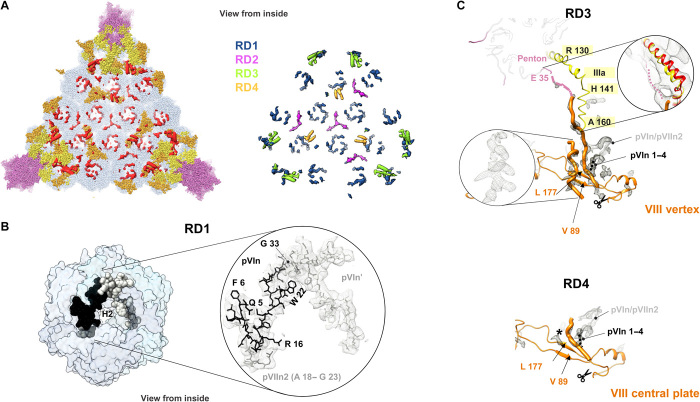
Fig. 6. Additional internal components of the HAdV-F41 capsid.
(A) Left: remnant densities in one facet (red), not occupied by traced proteins hexon (light blue), penton (light pink), IIIa (yellow), or VIII (orange). Right: remnant map components color-coded as RD1 to RD4 (see text). Densities correspond to the unsharpened map contoured at 1.5σ. (B) N-terminal peptides of proteins VI (pVIn, two copies) and VII (pVIIn2) tentatively traced in RD1, inside the hexon cavity. (C) Proposed interpretation of remnant densities RD3 and RD4. Except for the inset at the right hand side, RD in the sharpened map is shown, contoured at 2.5σ. Orange thin ribbon: traced part of the two copies of protein VIII (VIII vertex and VIII central plate). Orange thick worms: poly-Ala peptides modeled in the unassigned densities proposed to correspond to the central peptide of VIII. Left inset: possibly α-helical shape of one of the remnant densities in RD3. This density is much weaker in RD4, where a black star indicates its position. Scissors: AVP cleavage site in protein VIII. Peptide pVIn is represented as a gray ribbon, with the possible location of its four untraced N-terminal residues as a black dotted line. In the RD3 panel, the IIIa connecting helix is in yellow, the first residue traced in the penton base protein (E 35) is colored pink, and a dotted pink line indicates the possible trajectory of the untraced 34 residues. Right framed zoom: the IIIa connecting helix is shown overlapped with the HAdV-C5 helix in red, and the unsharpened map contoured at 1σ is shown as a gray mesh, to emphasize low-resolution density proposed to correspond to the penton base N-terminal arm.