Figure 1.
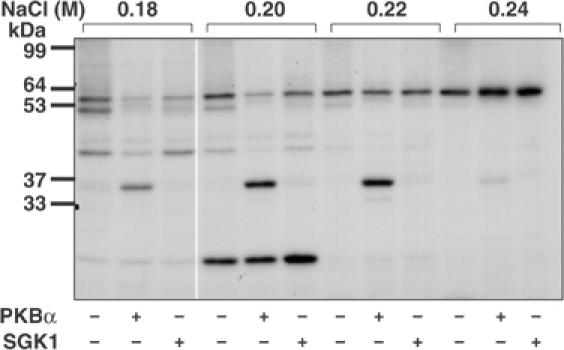
Detection of a 36 kDa substrate for PKBα in HeLa extracts. HeLa cell extracts were fractionated from 0–5% (w/v) PEG 6000, the 5% supernatant desalted and the material (950 mg protein) chromatographed on a 20 ml column of Source Q (see Materials and methods). Aliquots of each fraction were incubated for 4 min at 30°C as described previously (Knebel et al, 2001) at a further 10-fold dilution in the presence of 50 mM Tris–HCl pH 7.5, 1 mM EGTA, 0.1% (v/v) 2-mercaptoethanol, 10 mM MgCl2 and 20 nM [γ-32P]ATP (4 × 106 cpm/pmol) in the presence (+) or absence (−) of PKBα or SGK1, each at 0.4 U/ml (Davies et al, 2000); 1 U is the amount that catalyses the phosphorylation of 1 nmol substrate peptide in 1 min. After SDS–PAGE and autoradiography, a 36 kDa protein eluting at about 0.2 M NaCl was detected that was phosphorylated by PKBα, but very poorly by SGK1. Autophosphorylation of PKB and SGK1 was negligible under the conditions used.
