Figure 1.
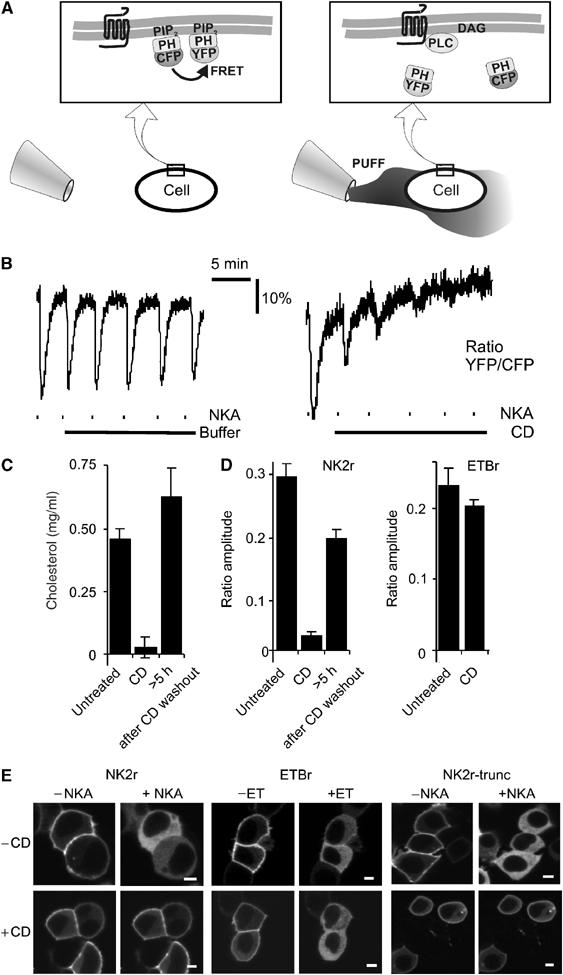
PIP2 hydrolysis induced by the NK2r, but not the ETB receptor, is cholesterol dependent. (A) Schematic representation of the FRET assay that allows for continuous read out of the integrity of the receptor–PLC–PIP2 signaling cascade. (Left panel) In a resting cell, CFP-PH and YFP-PH bind to PIP2 at the membrane and FRET occurs. (Right panel) Using a puffer pipette, the cell is briefly (∼10 s) exposed to agonist. This causes rapid degradation of PIP2, resulting in translocation of CFP-PH and YFP-PH into the cytosol, with consequent loss of FRET. Subsequently, the agonist dilutes out through diffusion, PIP2 is resynthesized and FRET recovers. For further details, see Materials and methods. (B) HEK293 cells expressing human NK2r were repeatedly stimulated with brief pulses of NKA (dashes, 10-s pulses from a puffer pipette containing 100 μM NKA). (Left panel) Control demonstrating repeated activation of the signaling cascade. (Right panel) Following a test pulse, CD (10 mM) was added (solid line), which rapidly inhibited signaling induced by further NKA pulses. (C) CD treatment causes depletion of cholesterol levels. Upon washout of CD, cholesterol levels recovered. (D) Quantitative analysis of the effects of CD treatment on agonist-induced FRET changes for NKA and ET. (E) HEK293 cells were cotransfected with GFP-PH and either the NK2r, the ETBr or a desensitization-defective truncation mutant of the NK2r as indicated. Confocal images were acquired from untreated (−CD) or CD-treated (+CD) cells before and 30 s after receptor stimulation. Scale bars, 5 μm. Note that in all cases, receptor stimulation induced translocation of GFP-PH to the cytosol (>80% of the cells), except for cells expressing NK2r that were treated with CD (wt NK2r, <30% of cells (partially) responded; desensitization-defective NK2r, <35% of cells responded; N>200; P<0.002).
