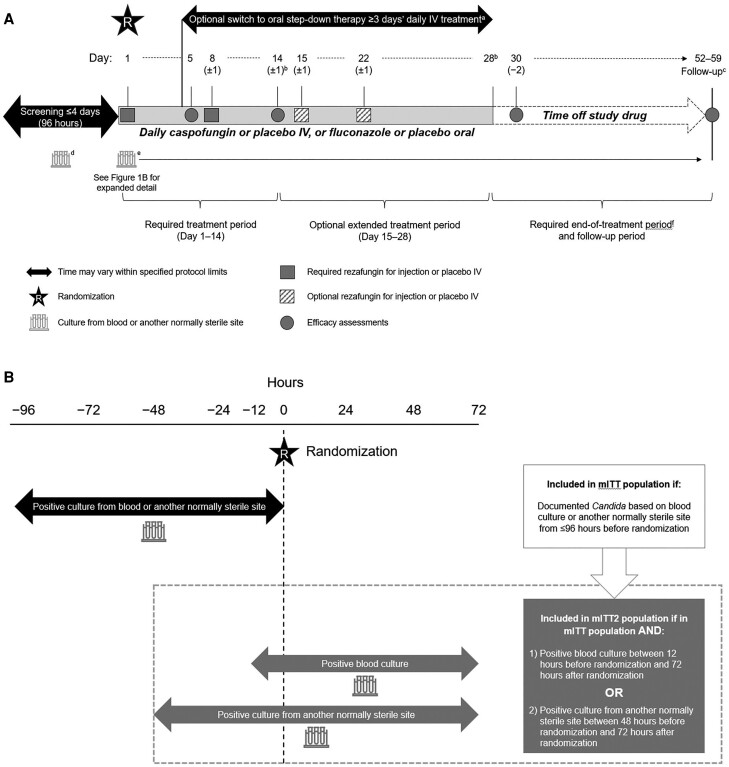
Figure 1.
A, ReSTORE study design. B, mITT and mITT2 population definitions. aAfter ≥3 days of IV study drug (or the minimum duration of IV therapy advised by the site's national/regional/local guidelines, whichever was greater), patients were permitted to switch to oral step-down therapy as long as all criteria specified in the protocol were met. Patients who were switched to oral step-down therapy could switch back to IV study drug therapy in the event of the development of a condition that prevented the subject from taking oral medication (eg, pancreatitis, urgent surgery), but were not permitted to switch back to IV study drug therapy for relapse of candidemia/IC or for intolerance or toxicity due to the study drug. bThe last required dose of the study drug was on day 14 and the last possible dose of the study drug was on day 28. cFollow-up occurred between days 52 and 59. Patients who stopped the study drug early (ie, clinical failures) and required a change in antifungal therapy to treat candidemia and/or IC were permitted to have an earlier follow-up visit occurring ≥30 days from the last weekly dose of IV rezafungin or IV placebo. dIf the positive blood culture used to qualify the patient for the study (ie, screening culture) was drawn >12 hours prior to randomization, an additional set of blood cultures was to be obtained ≤12 hours before randomization to determine whether patients were still candidemic at the time of enrollment. eBlood cultures were to be repeated daily (preferred) or every other day until the first negative blood culture result for Candida spp. with no subsequent positive culture (in cases when ≥1 samples were drawn and cultured after the first negative culture was available). fPatients completed an end-of-treatment visit ≤2 days after the last dose of study drug. All safety assessments were completed at the end-of-treatment visit. Efficacy assessments were also to be completed at the end-of-treatment visit. Abbreviations: IC, invasive candidiasis; IV, intravenous; mITT, modified intention-to-treat; mITT2, modified intention-to-treat 2.