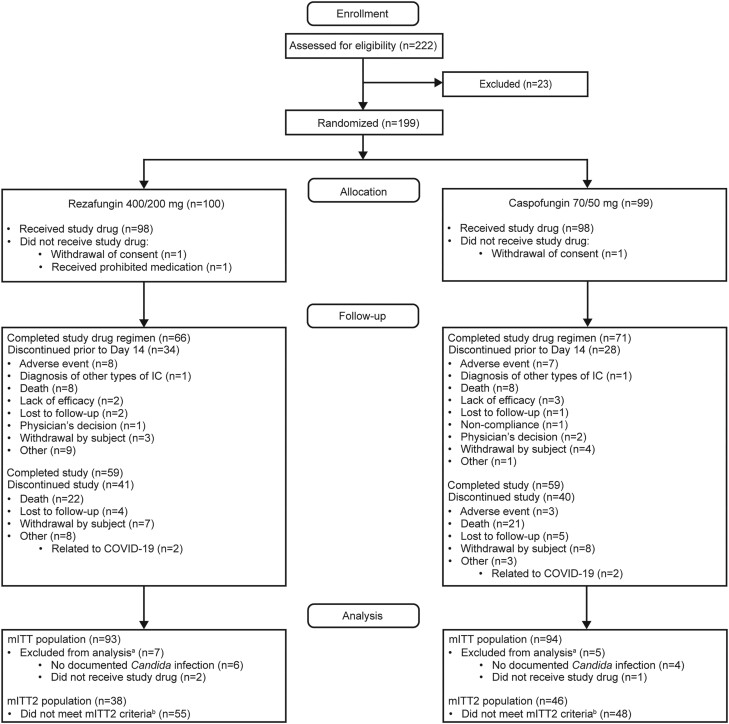
Figure 2.
Patient disposition in ReSTORE indicating mITT and mITT2 analysis populations. aSome patients met multiple reasons for exclusion and are included with each exclusion reason met. bPatients with either (1) a positive blood culture drawn between 12 hours before and 72 hours after randomization or (2) a positive culture from another normally sterile site sampled between 48 hours before and 72 hours after randomization and who received ≥1 dose of study drug were included in the mITT2 analysis. Figure modified from Thompson GR III et al. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): a multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet 2023; 401:49–59, with permission from Elsevier. ©2022 Elsevier Ltd. Abbreviations: COVID-19, coronavirus disease 2019; mITT, modified intention-to-treat; mITT2, modified intention-to-treat 2.