Figure 3:
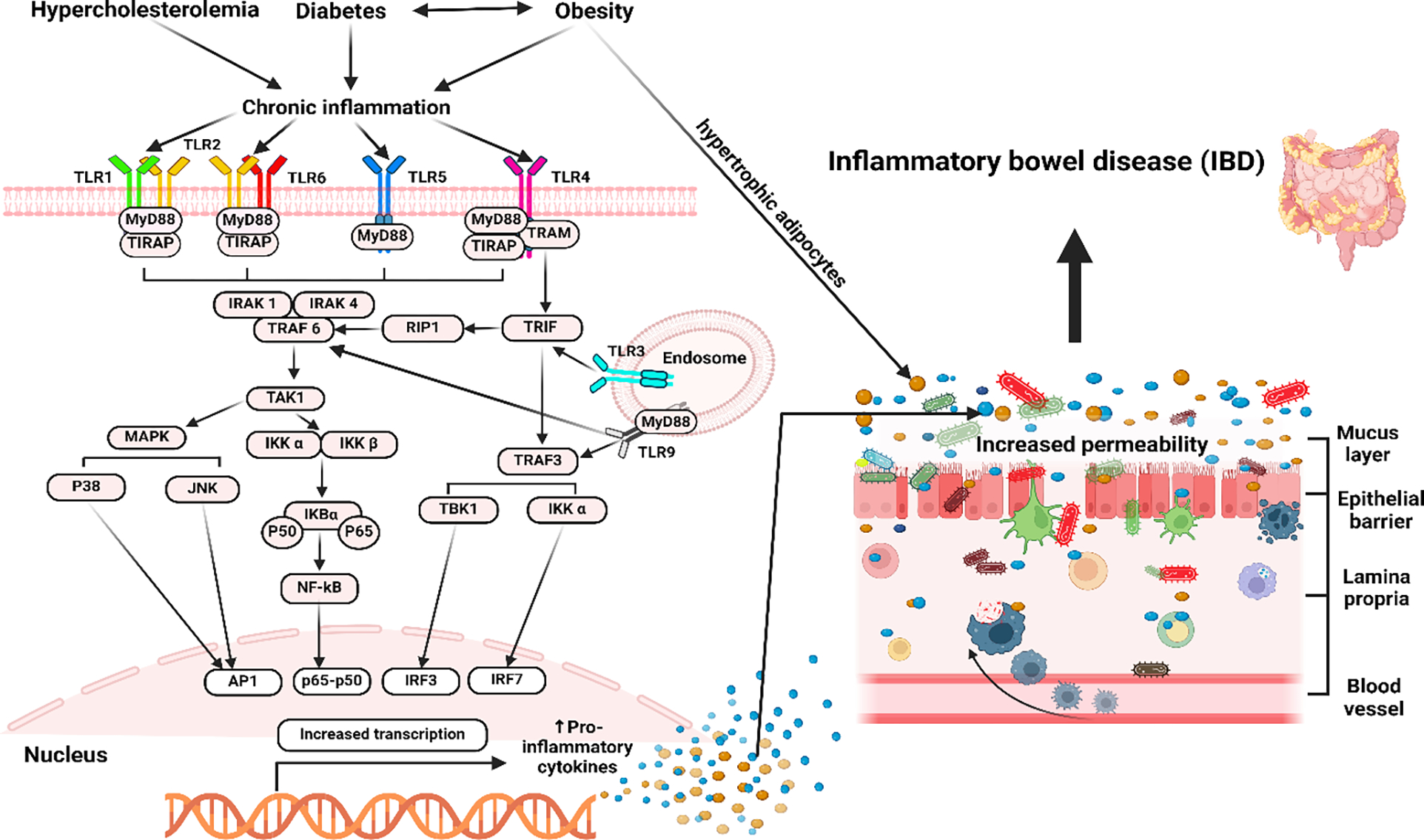
The illustration above highlights the role of TLRs and the process of their activation in DM and its subsequent correlation with IBD. Diabetes, especially T2DM, and obesity are characterized by persistent low-grade inflammation, which plays a crucial role in the etiology and development of these conditions. Hyperglycemia, high FFA levels, oxidative stress, and other conditions trigger mostly TLR2 and TLR4 activation, which in turn promotes the creation of several transcriptional factors that lead to the generation of pro-inflammatory cytokines and inflammation. Inflammation and the subsequent production of cytokines in the gut result in dysbiosis, which leads to increased permeability of the intestines, sometimes referred to as “leaky gut” by impairing epithelial tight junctions and facilitating the translocation of microbial products like lipopolysaccharide (LPS) into the bloodstream, which in turn causes systemic inflammation. Increased concentrations of circulating LPS are linked to the development of insulin resistance. Dysbiosis can initiate abnormal immunological reactions, resulting in an excessive release of pro-inflammatory cytokines that can contribute to the development of IBD; therefore, implying a connection between diabetes, obesity, and IBD.
