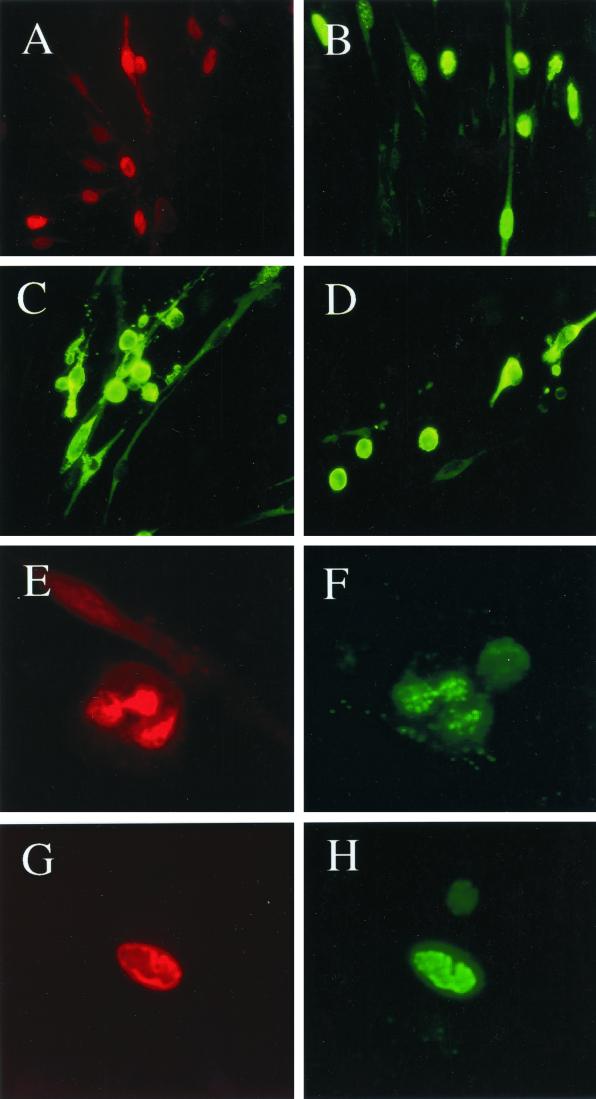
FIG. 5.
Development of clusters of infected DMVEC displaying late lytic CPE and containing active viral DNA replication compartments. (A) Expression of the KSHV-encoded ORF59 protein including typical late stage herpesvirus nuclear DNA replication compartments within a clustered miniplaque in a 10-day confluent secondary JSC1 spindle cell culture, as detected by IFA with mouse anti-ORF59 MAb (red rhodamine label, 63× objective). (B) Expression of ORF-K8 including nuclear DNA replication compartment-associated forms within a clustered miniplaque in a 10-day confluent secondary JSC1 spindle cell culture, as detected by IFA with rabbit anti-K8 PAb (green fluorescein isothiocyanate [FITC] label; 63× objective). (C and D) Expression of the KSHV-encoded lytic membrane protein ZMP-A in small plaque-like patches of rounded late stage lytic cycle-infected DMVEC cells displaying CPE. Two different fields within a 10-day secondary JSC1 spindle cell culture detected by IFA with rabbit anti-ZMP-A PAb (green FITC label; 63× objective) are shown. (E and F) Colocalization of ORF59 and ORF-K8 by double-label IFA in viral DNA replication compartments in a late lytic stage JSCI-infected DMVEC cell, showing rounding and CPE, as detected with mouse anti-ORF59 MAb (E; red rhodamine label) and rabbit anti-ORF8 PAb (F; green FITC label) in the same field (100× objective). (G and H) Colocalization of newly synthesized DNA (BUdR pulse-label) and the ORF-K8 nuclear protein in a mature viral DNA replication compartment in a late lytic stage rounded JSC1-infected DMVEC cell as detected by double-label IFA using mouse anti-BUdR MAb (G; red rhodamine label) and rabbit anti-K8 PAb (H; green FITC label) in the same field (100× objective).