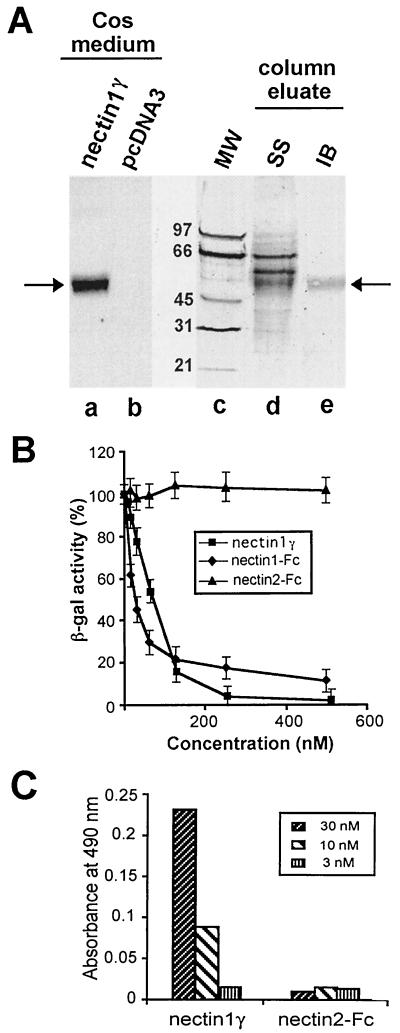
FIG. 3.
(A) Purification of nectin1γ from culture medium of transfected Cos1a cells. To produce and affinity purify nectin1γ, R1.302 IgGs were precipitated overnight at 4°C from R1.302 ascitic fluid with ammonium sulfate (50% saturated) and then conjugated to CNBr-activated Sepharose 4B (Amersham Pharmacia Biotech AB) according to the manufacturer's instructions. The column was loaded with about 1 liter (concentrated using Microcon YM10 filters [Millipore]) of culture medium from Cos1a cells transfected with pCDSR1.D1 plasmid, harvested 6 days after transfection. The concentrated medium was allowed to adsorb overnight at 4°C and eluted with 3 M potassium thiocyanate in equilibration buffer (10 mM Tris-HCl [pH 7.5], 0.5 M NaCl). Pooled protein fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining (SS) (lane d) or by immunoblotting (IB) with an antipeptide serum directed against the C terminus of nectin1γ (lane e). Lanes a and b illustrate the specificity of the antipeptide immune serum. Shown is the immunoblot reactivity of the serum to the medium of Cos1a cells transfected with pCDSR1.D1, encoding nectin1γ, or with the pcDNA3 vector alone. The migration positions of the molecular weight markers (MW) are shown in lane c. Arrows point to the nectin1γ secreted in the culture medium of transfected Cos1a cells and in the affinity column eluate. (B) Inhibition of HSV-1 R8102 infectivity by nectin1γ and nectin1-Fc. R8102 virions were preincubated with increasing concentrations of the partially purified nectin1γ, nectin1-Fc, or nectin2-Fc for 1 h at 37°C. Virions were then allowed to infect cells expressing nectin1β, grown in 96-well plates, for 2 h at 4°C. Infection was quantified as β-Gal expression by ONPG staining. Each point represents the average of three values. The concentration of nectin1γ was estimated by multiplying the actual concentration by 0.3 to take the partial purification shown in panel A into consideration. (C) Binding of nectin1γ to virions. The wells in 96-well plates were coated with gradient-purified R7032 virions (10) and reacted with nectin1γ at the indicated concentrations, followed by the rabbit antipeptide serum (1:400), and peroxidase-conjugated anti-rabbit IgG (17). As a background control, R7032 virions were reacted with nectin2-Fc, a recombinant form of nectin2, in which the ectodomain is fused to the Fc portion of human IgGs (12). Binding was detected with peroxidase-conjugated anti-human IgG.