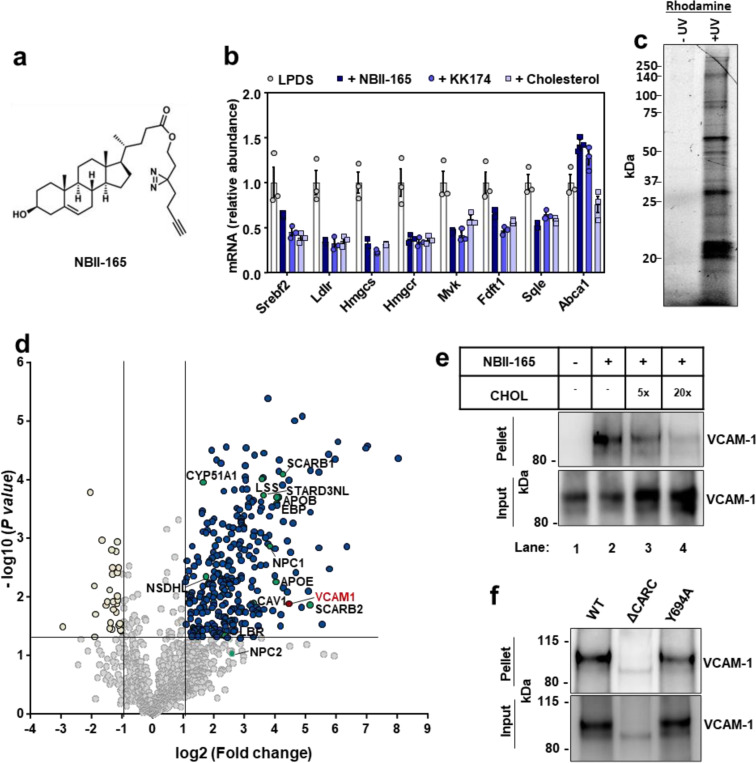
Fig. 1. Cholesterol-mimetic probes identify VCAM-1 as a cholesterol binding protein in primary human ECs.
(a) Structure of NBII-165 probe. (b) HUVECs were depleted of cholesterol overnight in LPDS containing simvastatin. Cells were then loaded with 35 μM NBII-165, KK-174 or cholesterol complexed to methyl-beta cyclodextrin for 4 h before collection and assessment of SREBP-2 targets by qPCR. (c) Rhodamine-azide signal in HUVEC lysates. HUVECs were incubated with 10 μM NBII-165 probe for 1 h, with and without 365 nm UV irradiation before attachment of a rhodamine-azide fluorophore by click chemistry and separation of proteins by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). The in-gel rhodamine signal was visualized with a fluorescent imager. (d) Volcano plot showing proteins that were detected by mass spec after immunoprecipitation of NBII-165-bound proteins. Dark blue dots indicate significantly enriched proteins. Yellow dots indicate proteins that were significantly lower in the UV exposed samples. Green dots indicate known sterol binding proteins. Burgundy dot indicates VCAM-1. (e) Competition assay showing that cholesterol competes with NBII-165 for binding to VCAM-1 in HUVECs stably overexpressing human VCAM-1. Input shows VCAM-1 detected in whole cell lysates prior to immunoprecipitation and pellet shows VCAM-1 detected after streptavidin immunoprecipitation of probe bound proteins. (f) Immunoprecipitation of WT VCAM-1 or mutant versions of VCAM-1 either lacking the CARC motif or with a tyrosine for alanine mutation at amino acid 694 after incubating HUVECs with KK-174 followed by UV crosslinking. Data are represented as mean ± SEM.