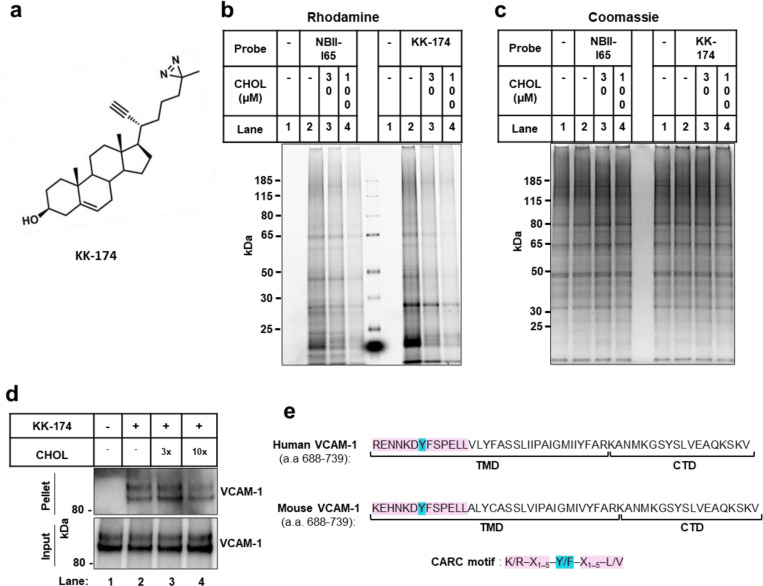
Extended Data Fig. 1. Cholesterol directly binds to VCAM-1.
(a) Structure of the KK-174 probe. (b and c) The first lane in each condition represents cells with no cholesterol probe added before UV exposure. The remaining lanes represent cells loaded with 10 μM cholesterol-mimetic probe alone or with increasing concentrations of MβCD-cholesterol (30 μM or 100 μM) for 1 h before UV crosslinking. After UV exposure, cells were lysed, a rhodamine-azide tag was conjugated via click chemistry onto probe-bound samples, and cellular proteins were separated by SDS-PAGE. (b) Probe bound samples were visualized via the florescent rhodamine signal. (c) Coomassie staining of total cellular proteins from (b). (d) Competition assay showing that cholesterol competes with KK-174 for binding to VCAM-1 in HUVECs stably overexpressing human VCAM-1. Input shows VCAM-1 detected in whole cell lysates prior to immunoprecipitation and pellet shows VCAM-1 detected after streptavidin immunoprecipitation of probe bound proteins. (e) Amino acids 688–739 (corresponding to the TMD and CTD) in human and mouse VCAM-1 with the CARC motif in the TMD highlighted in pink and the central tyrosine (Y) residue that was mutated in Fig. 1F highlighted in blue.