FIG. 6.
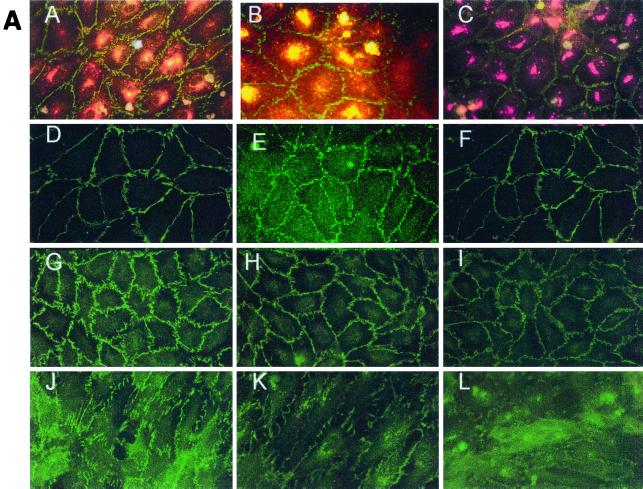
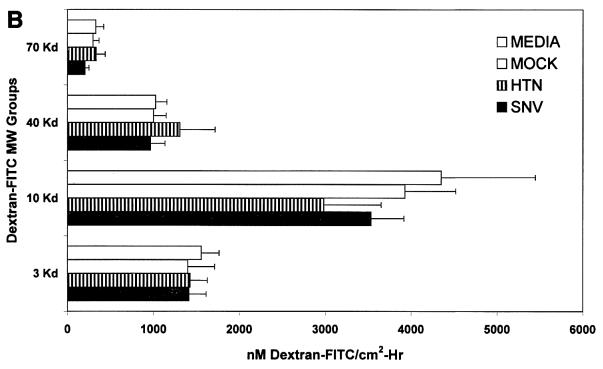
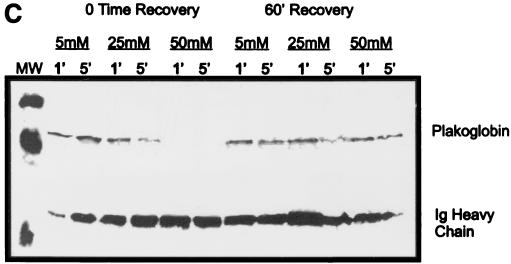
Effect of hantavirus infection on the establishment and maintenance of functional permeability barriers. (A) Indirect immunofluorescence of the organization and distribution of HMVEC-L junctional integral proteins: ZO-1 in SNV-infected (A), mock-infected (D), uninfected (G), or uninfected TNF-α (24 h)-treated (J) cells; CAD-5 in SNV-infected (B), mock-infected (E), uninfected (H), or uninfected TNF-α (24 h)-treated (K) cells; and CD31 in SNV-infected (C), mock-infected (F), uninfected (I), or uninfected TNF-α (24 h)-treated (L) cells. (B) Measurements of paracellular and transcellular permeability in confluent cultures of SNV-infected, HTN-infected, mock-infected, or uninfected HMVEC-Ls. Fluorescently labeled dextran particles of 10, 40, and 70 kDa were used for paracellular permeability measurements, and 3-kDa dextran particles were used for transcellular permeability measurements, as described in the text. Paracellular permeability was indirectly proportional to the molecular weight of the dextran particles. (C) Measurements of the formation of adherens junctions in uninfected HMVEC-Ls. After experimental disruption by treatment with EDTA, adherens junction complexes in confluent HMVEC-L monolayers were allowed to reform in the presence of complete medium. CAD-5 complexes immunoprecipitated from cell lysates were resolved by SDS-polyacrylamide gel electrophoresis, and the presence of plakoglobin in reformed adherens complexes was determined by Western blotting. (D) Measurements of the reestablishment of functional permeability barriers in confluent cultures of SNV-infected, HTN-infected, mock-infected, or uninfected HMVEC-Ls after experimental disruption of adherens junction complexes by treatment with EDTA.