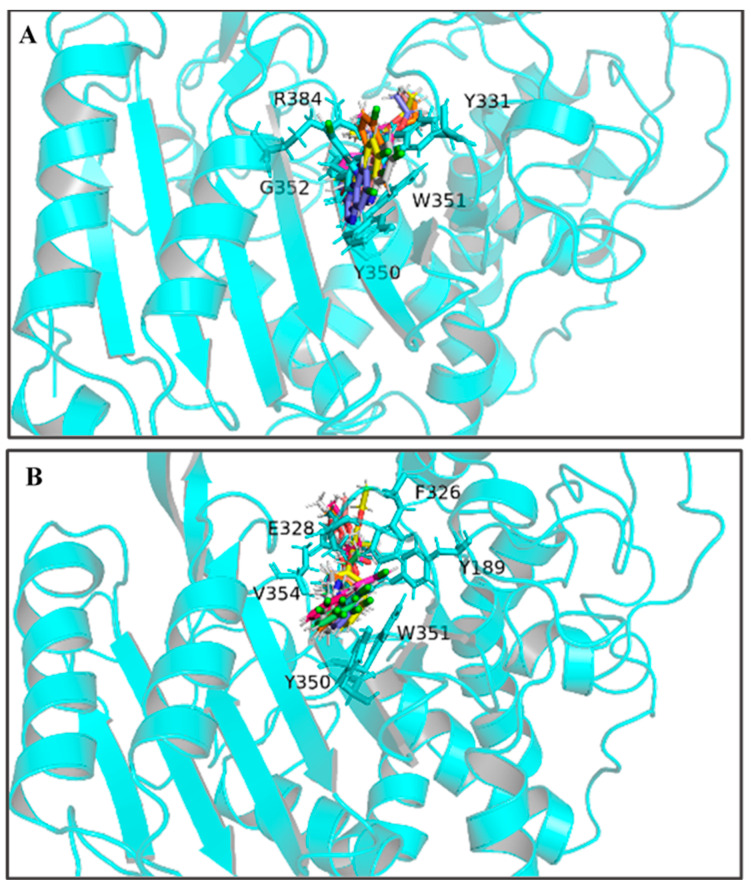
Figure 4.
The ten representative snapshots of (R)-QE (A) and (S)-QE (B) superposed at their respective QeH active sites inside the interior of hydrophobic pocket during their MD runs. Key residues of QeH and two ligands were represented by stick models, and the residues (Tyr331, Tyr350, Trp351, Gly352 and Arg384 for the QeH-(R)-QE complex; Tyr189, Phe326, Glu328, Tyr350, Trp351 and Val354 for the QeH-(S)-QE complex) with their respective binding affinities over −1.0 kcal·mol−1 were marked by black labels.