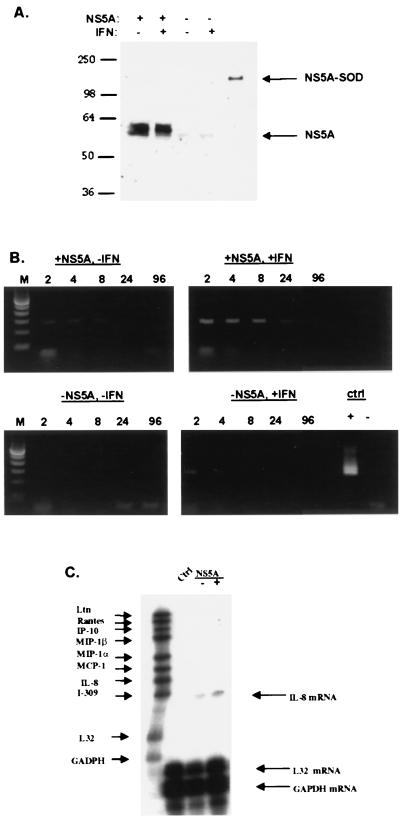
FIG. 3.
NS5A expression increases the expression of IL-8 mRNA. (A) Tetracycline-regulated expression of NS5A in human cells. HeLa cells expressing FL-NS5A-NR were incubated in medium with or without tetracycline for 48 h to repress or induce NS5A expression, respectively. Cells were then treated or not treated with IFN (20 U/ml) for 24 h. Cell extracts were prepared, and equal amounts of total cellular proteins were analyzed by Western blotting. The positions of the 58-kDa NS5A and positive control, NS5-(superoxide dismutase [SOD]), proteins are shown with arrows. (B) Semiquantitative detection of IL-8 mRNA by RT-PCR. cDNA was prepared from HeLa cells expressing or not expressing FL-NS5A-NR and treated or not treated with IFN. Serial dilutions of 1:2, 1:4, 1:8, 1:24, and 1:96 of cDNA (depicted above each lane with 2, 4, 8, 24, and 96, respectively) were subjected to PCR using IL-8-specific primers. Positive and negative controls (ctrl) are depicted with + and − signs, respectively. Amplification of cDNA generated without reverse transcriptase produced no PCR signal, indicating that the observed IL-8 PCR products were derived from IL-8 mRNA. Lanes M, molecular size markers. (C) Detection of IL-8 mRNA by RNase protection assay. Equal amounts of total cellular RNA from HeLa cells expressing or not expressing FL-NS5A-NR were hybridized to a 32P-labeled riboprobe cocktail containing probes for various human chemokines, including IL-8. Hybrids resistant to RNase digestion were separated on denaturing acrylamide gels, and autoradiography was performed. The positions of IL-8, control L32, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs are indicated with arrows. Note that the size of the protected fragment is smaller than the probe size because the probe contains vector-derived sequences that do not hybridize with target mRNA.