Abstract
This study aimed to characterize Pseudomonas aeruginosa strains isolated from hospitalized patients during the COVID-19 pandemic. This was achieved using phenotypic and molecular techniques, including their antimicrobial resistance profile and biofilm formation. Eighteen strains were isolated from a hospital in Rio de Janeiro, Brazil, and identified by VITEK®2, MALDI-TOF/MS (VITEK MS® and MALDI Biotyper®), and 16S rRNA sequencing. Fourier-transform infrared (FTIR) spectroscopy, antimicrobial susceptibility testing, and biofilm formation and disinfectant tolerance tests were applied to evaluate the virulence characteristics of the strains. VITEK®2 (≥99%), VITEK MS® (≥82.7%), and MALDI Biotyper® (score ≥ 2.01) accurately identified the P. aeruginosa strains, but 16S rRNA sequencing did not differentiate the species P. aeruginosa from P. paraeruginosa. FTIR typing identified three different clusters, but no correlation between the phenotypical or antimicrobial susceptibility testing patterns was found. Most strains exhibited resistance to various antimicrobials. The exceptions were sensitivity to amikacin and norfloxacin, and consequently, these could be considered potential treatment options. Most strains (n = 15, 83.3%) produced biofilms on polystyrene. Sodium hypochlorite treatment (0.5%/15 min) was shown to be the most effective disinfectant for biofilm elimination. P. aeruginosa biofilm formation and tolerance to disinfectants demonstrate the need for effective cleaning protocols to eliminate contamination by this organism in the hospital environment and medical equipment.
Keywords: P. aeruginosa, COVID-19, multidrug-resistant
1. Introduction
Pseudomonas aeruginosa is a Gram-negative pathogen commonly associated with healthcare-associated infections (HAIs), causing infections, especially in immunocompromised patients [1]. It is mainly associated with respiratory tract infections, as well as ventilator-associated pneumonia (VAP), meningoencephalitis, and sepsis [2]. Due to its remarkable capacity to resist antibiotics, the eradication of this pathogen has become a challenge. P. aeruginosa is intrinsically resistant to several antimicrobials, such as kanamycin and neomycin [3]. It has adaptive resistance, which includes biofilm-mediated resistance and the acquisition of resistance genes through horizontal gene transfer [4]. The quick identification of pathogens in the routine of the microbiology laboratories would speed up the procedures of infection control that contribute to the elimination of P. aeruginosa in the hospital environment [5]. Fourier-transform infrared (FTIR) spectroscopy (Bruker Optics-Daltonics, Bremen, Germany), performed with IR Biotyper, is a method that aims to type microorganisms within 3 h [6]. The technique generates spectra based on the absorption of infrared light by the different cell chemical components, providing biochemical fingerprints of the bacterial cells [5]. Since the cell wall composition, membrane structure, and intracellular components vary between strains, each isolate will exhibit a unique FTIR spectrum [7]. The FTIR spectra generated can be compared so that strains can be differentiated and profiled according to their spectral signatures [7]. This technique has been used for the surveillance of several nosocomial Gram-negative rods, including P. aeruginosa [5,6,8].
Previous studies have reported the prevalence of 12% P. aeruginosa in co-infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Furthermore, hospital outbreaks caused by this pathogen have been reported around the world [9,10]. The ability to form a biofilm on medical devices used in endotracheal intubation and mechanical ventilation is likely to be one of the main reasons for the persistence of P. aeruginosa [11]. An increase in antibiotic prescriptions was also observed during the COVID-19 pandemic. This could result in the emergence of multidrug-resistant (MDR) P. aeruginosa strains and make therapeutic management more difficult [12]. It is plausible that P. aeruginosa modifies its virulence factors during co-infection with SARS-CoV-2, leading to greater antimicrobial resistance and biofilm formation [13].
This study aimed to characterize P. aeruginosa strains isolated from hospitalized patients during the COVID-19 pandemic using phenotypic and molecular techniques and evaluate their antimicrobial resistance profile and biofilm formation and its tolerance to disinfectants.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
Eighteen strains identified as P. aeruginosa using VITEK®2 (bioMérieux, Craponne, France), with a confidence of > 99%, were isolated between July 2021 to March 2022 from a hospital located in Rio de Janeiro State, Brazil. The strains were isolated from tracheal secretion (n = 9), urine (n = 6), rectal swab (n = 2), and oral swab (n = 1) (Table 1). The strains were isolated from patients with COVID-19 and non-COVID-19 co-infection, and from surveillance samples collected from the patients by the Hospital Infection Prevention and Control Committee (HIPCC).
Table 1.
Details of P. aeruginosa strains (n = 18) isolated in a Brazilian hospital during the COVID-19 pandemic.
| Strain ID | Source | Patient ID | Local | Origin | Date | MALDI-TOF MS a | |
|---|---|---|---|---|---|---|---|
| VITEK MS® (%) b | MALDI Biotyper® (Score) c | ||||||
| PS001.21 | Urine | A | ICU d-1 | COVID-19 e | 07/12/2021 | P. aeruginosa (99.9) | P. aeruginosa (2.40) |
| PS002.21 | Urine | B | ICU-1 | COVID-19 | 07/14/2021 | P. aeruginosa (99.9) | P. aeruginosa (2.05) |
| PS003.21 | Tracheal secretion | C | ICU-1 | COVID-19 | 07/23/2021 | P. aeruginosa (84.0) | P. aeruginosa (2.27) |
| PS005.21 | Tracheal secretion | D | ICU-1 | COVID-19 | 07/30/2021 | P. aeruginosa (99.9) | P. aeruginosa (2.03) |
| PS006.21 | Tracheal secretion | E | ICU-1 | COVID-19 | 07/28/2021 | P. aeruginosa (99.9) | P. aeruginosa (2.31) |
| PS007.21 | Urine | F | ICU-1 | COVID-19 | 08/06/2021 | P. aeruginosa (99.9) | P. aeruginosa (2.04) |
| PS008.21 | Oral swab | G | ICU-1 | COVID-19 | 08/06/2021 | P. aeruginosa (95.1) | P. aeruginosa (2.03) |
| PS010.22 | Rectal swab | H | ICU-2 | HIPCC f | 01/27/2022 | P. aeruginosa (99.9) | P. aeruginosa (2.33) |
| PS011.22 | Tracheal secretion | I | Medical Clinic g-1 | Non-COVID-19 | 02/17/2022 | P. aeruginosa (92.7) | P. aeruginosa (2.00) |
| PS012.22 | Rectal swab | J | ICU-2 | HIPCC | 02/23/2022 | P. aeruginosa (99.9) | P. aeruginosa (2.31) |
| PS013.22 | Urine | K | ICU-2 | Non-COVID-19 | 02/28/2022 | P. aeruginosa (96.1) | P. aeruginosa (2.22) |
| PS014.22 | Urine | L | ICU-2 | COVID-19 | 03/01/2022 | P. aeruginosa (82.7) | P. aeruginosa (2.34) |
| PS015.22 | Tracheal secretion | M | ICU-1 | COVID-19 | 02/24/2022 | P. aeruginosa (91.6) | P. aeruginosa (2.00) |
| PS016.22 | Tracheal secretion | N | ICU-2 | Non-COVID-19 | 03/05/2022 | P. aeruginosa (92.0) | P. aeruginosa (2.04) |
| PS017.22 | Tracheal secretion | O | ICU-1 | COVID-19 | 03/04/2022 | P. aeruginosa (99.4) | P. aeruginosa (2.26) |
| PS018.22 | Tracheal secretion | C | ICU-1 | COVID-19 | 03/09/2022 | P. aeruginosa (99.9) | P. aeruginosa (2.10) |
| PS019.22 | Tracheal secretion | M | ICU-1 | COVID-19 | 03/13/2022 | P. aeruginosa (99.9) | P. aeruginosa (2.01) |
| PS020.22 | Urine | P | Medical Clinic-2 | Non-COVID-19 | 03/17/2022 | P. aeruginosa (99.9) | P. aeruginosa (2.07) |
a—matrix-assisted laser desorption ionization time-of-flight mass spectrometry performed; b—results analyzed by SARAMIS Premium software v. 4.0.0.14, only strains with a percentage ≥ 75% similarity were considered identified; c—results analyzed by MBT Compass software. Data interpretation was done in line with Bruker’s standard criteria; a species cut-off score value of 2.0 and a genus cut-off score value of 1.7 were applied; d—intensive care unit; e—patient with co-infection with severe acute respiratory syndrome coronavirus 2; f—Hospital Infection Prevention and Control Committee; g—medical clinic.
P. aeruginosa ATCC 27,853 was used as the control culture for specific tests.
Stock cultures were prepared and maintained at −70 °C in Difco™ Skim Milk 10% (BD Biosciences, Le Pont de Claix, Auvérnia-Ródano-Alpes, France), containing 30% glycerol (Merck, Darmstadt, Germany). The inocula were prepared by transferring one loopful into 3 mL fresh brain-heart infusion broth (BHI) (Merck, Darmstadt, Germany) and incubating at 37 °C for 24 h. For daily use, cultures were maintained at 5 °C on Tryptic Soy Agar (TSA) (BioCen do Brasil, São Paulo, Brazil). All strains were deposited at the Coleção de Bactérias do Ambiente e Saúde (CBAS) hosted at Fundação Oswaldo Cruz (Fiocruz), Rio de Janeiro, Brazil (https://portal.fiocruz.br/, acessed on 15 August 2024). CBAS is affiliated with the World Federation for Culture Collections (WFCC) and registered as the World Data Centre for Microorganisms (WDCM) 958 (https://ccinfo.wdcm.org/details?regnum=958, acessed on 15 August 2024).
2.2. Identification by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry Proteomic Characterization
The isolates were grown on Sheep Blood Agar (SBA) (BioCen do Brasil, São Paulo, Brazil) at 32.5 °C for 48 h. All strains were identified using MALDI Biotyper® (Bruker Daltonics, Bremen, Germany) and VITEK MS® systems (bioMérieux, Craponne, Lyon, France) according to the manufacturer’s instructions. For VITEK® MS, using the SARAMIS Premium (version 4.0.0.14) program, the strain was considered identified when >75% similarity was obtained. For the MALDI Biotyper®, the MBT Compass Library Revision K (2022) was used and the confidence range of 0.00–1.69 meant that no identification was possible for the microorganism, at 1.70–1.99 the identification was of low confidence at the species level, and at 2.00–3.00 the identification was of high confidence at the species level. The strains not identified to species level by both MALDI-TOF MS equipment were subjected to complete 16S rRNA gene sequencing described below.
2.3. 16S rRNA Gene Sequencing Analysis
The isolates were identified by 16S rRNA gene Sanger sequencing analysis using the MicroSEQ™ Full Gene 16S rDNA kit (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. The sequences were processed using DNA Star LaserGene SeqMan software v. 7.0.0, and identification results were obtained from the website https://www.ezbiocloud.net/ (Database Update: 7 July 2021, accessed on 23 February 2023). Only species that presented an identification ≥ 98.7% were considered valid [14]. All sequences were deposited at https://www.ncbi.nlm.nih.gov/, acessed on 15 August 2024, and the access numbers are provided in Table 2. The sequences of the strains: P. aeruginosa JCM5962 (access number BAMA01000316), P. paraeruginosa PA7 (access number ON359917), P. otitidis MCC10330 (access number AY953147), P. lalkuanensis PE08 (access number MF943158), and Luteimonas marina FR1330 (access number EU295459), were included in the phylogenetic relationship of these strains. A phylogenetic tree based on multiple alignments of nearly complete 16S rRNA gene sequences was constructed using the neighbor-joining and the ClustalW algorithm with the software MEGA 11 by employing the Tamura two-parameter model with branch support based on 1000 bootstrap [15].
Table 2.
16S rDNA sequencing analysis of P. aeruginosa strains (n = 18).
| Strain ID | NCBI a Access Number | Base Pair Length | Identification (%) |
|---|---|---|---|
| PS001/21 | OQ944132 | 1027 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS002/21 | OQ944133 | 856 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS003/21 | OQ944134 | 858 | P. aeruginosa (99.30)/P. paraeruginosa (99.30) |
| PS005/21 | OQ945448 | 873 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS006/21 | OQ945449 | 928 | P. aeruginosa (99.78)/P. paraeruginosa (99.78) |
| PS007/21 | OR041497 | 1337 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS008/21 | OQ945450 | 849 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS010/22 | OQ945451 | 874 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS011/22 | OR041498 | 1333 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS012/22 | OQ945452 | 873 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS013/22 | OQ945453 | 588 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS014/22 | OQ945454 | 1016 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS015/22 | OQ945455 | 975 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS016/22 | OQ945456 | 922 | P. aeruginosa (99.89)/P. paraeruginosa (99.89) |
| PS017/22 | OQ945457 | 1003 | P. aeruginosa (99.90)/P. paraeruginosa (99.90) |
| PS018/22 | OQ945458 | 935 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS019/22 | OQ945459 | 905 | P. aeruginosa (100)/P. paraeruginosa (100) |
| PS020/22 | OQ945460 | 1025 | P. aeruginosa (99.41)/P. paraeruginosa (99.41) |
a—National Center for Biotechnology Information.
2.4. Typing by FTIR Spectroscopy Analysis
Isolates were cultured on TSA at 37 ± 1 °C for 24 h. Colonies were selected and 1 μL of the colony was mixed well in a suspension vial containing 70% ethanol (50 μL), followed by the addition of 50 μL distilled water and mixing. Three spots (15 μL each) were loaded onto a 96-well silicon microplate. The completely dried plate was mounted on the IR Biotyper® spectrometer (Bruker Optics-Daltonics, Bremen, Germany). Test standards (IRTS1 and IRTS2), spotted in duplicate, were also included. A dendrogram was created with the raw data to cluster the separation spectrum. The cut-off value on the dendrogram was automatically calculated using OPUS v.7.5 software (Bruker Optics-Daltonics GmbH, Bremen, Germany). The distance matrix based on the average Euclidean distance and linkage algorithm reflects the separation distances of all strains.
2.5. Antimicrobial Susceptibility Profile
P. aeruginosa isolates were evaluated for susceptibility to antimicrobial agents using the disk diffusion agar method, following the criteria of the Clinical and Laboratory Standards Institute (CLSI) (2020) [16]. Antimicrobials tested were sulfamethoxazole-trimethoprim (SXT), meropenem (MEM), ceftazidime (CAZ), cefoxitin (FOX), cefepime (CEF), ceftriaxone (CRO), cefuroxime axetil (CXM), piperacillin-tazobactam (TZP), amikacin (AMI), gentamicin (GEN), ciprofloxacin (CIP), norfloxacin (NOR), and imipenem (IPM), and interpreted following the CLSI [14]. The strains were classified as MDR, extensively drug-resistant (XDR), or pandrug-resistant (PDR) according to the criteria proposed by Magiorakos et al. [17]. The author characterizes MDR as non-susceptible to ≥1 agent in ≥3 antimicrobial classes, XDR as non-susceptible to ≥1 agent in all but ≤2 categories, and PDR as non-susceptible to all antimicrobial agents in all antimicrobial classes [17].
2.6. Biofilm Formation and Its Tolerance to Disinfectants
The strains were tested for biofilm formation in polystyrene surfaces as described by Vasconcellos et al. [18]. The strains were grown on tryptic agar soy (TSA) at 37 °C for 24 h. Colonies were selected and cultured on 3 mL of BHI (Merck, Darmstadt, Germany) at 37 °C for 24 h. A total of 200 µL of the culture was inoculated in triplicate on a polystyrene surface in 96 well plates (Falcon®, Elizabeth, NJ, USA) and incubated at 22.5 ± 2.5 °C and 37 ± 2 °C for 48 h. The plates were washed five times with distilled water and kept at room temperature until completely dry. An aliquot (200 µL) of 0.41% crystal violet solution (Merck, Darmstadt, Germany) was added to each well and the microplates were kept at room temperature for 45 min. The wells were aspirated, and the microplates were washed five times with water and kept at room temperature until completely dry. Then, 200 µL of 96% absolute ethanol (Merck, Darmstadt, Germany) was added to each well and the microplates were kept under agitation. After 10 min, 150 µL of the contents of each well was transferred to new microplates, and the optical density (O.D.) was read in a spectrophotometer (bioMérieux, Reader 270, Craponne, France).
The assay was repeated in triplicate in three separate experiments for each strain. P. aeruginosa ATCC 27,853 was used as positive control. Strains were categorized based on the cut-off optical density (ODC) compared with OD of the negative control: non-adherent (−) OD ≤ ODC, weakly adherent (+) ODC < OD ≤ 2 × ODC, moderately adherent (++) 2 × ODC < OD ≤ 4 × ODC, or strongly adherent (+++) 4 × ODC < OD [19]. The final categorization was the medium of the nine results.
The strains classified as moderately or strongly adherent were selected to evaluate their tolerance to the following disinfectants: alcohol 70% for 5 min (Merck, Darmstadt, Germany), sodium hypochlorite 0.1% and 0.5% for 15 min (Brasquímica, Belo Horizonte, Brazil), disinfectant based on synergistic association between ammonium quaternary 5th generation and stabilized polymeric biguanide/10 min (Mirax BG diluted 1:200, Hortolândia, Brazil), and peracetic acid 0.5%/10 min (Divosan Forte VT6, Diversey®, Peróxidos do Brasil Ltd.a, Curitiba, Brazil). Differences in the degree of biofilm and its tolerance to disinfectants were examined by Wilcoxon signed ranks test using software R Core Team v. 4.2.0 (Vienna, Austria). p-values < 0.05 were considered significant.
3. Results
3.1. Identification and Typing
VITEK MS® and MALDI Biotyper® identified all strains as P. aeruginosa with ≥82.7% confidence and score ≥ 2.00, respectively (Table 1). According to 16S rRNA gene sequencing analysis, the strains were identified as P. aeruginosa with P. paraeruginosa as a possibility (Table 2). The neighbor-joining tree clustered the strains together (Figure 1).
Figure 1.
Neighbor-joining tree based on partial 16S rRNA gene sequences (549 bp) showing the phylogenetic position of the strains evaluated in the present study (n = 18). The numbers at the nodes indicate the percentage of 1000 bootstrap replicates; only values > 50% are shown. Luteimonas marina FR1330 was used as an outgroup. The scale bar represents 0.02 substitutions per nucleotide position. GenBank accession number is given.
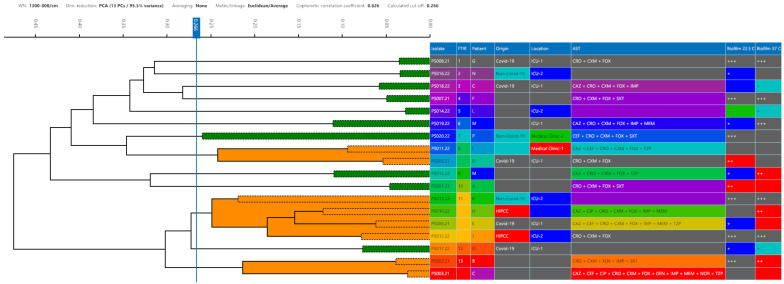
The typing of the 18 isolates obtained from FTIT resulted in 13 different profiles with a 0.266 cut-off (Figure 2). The FTIR profile 11 formed the largest cluster with four strains (PS006, PS010, PS012, and PS013), followed by profiles 8 (PS005 and PS011) and 13 (PS002 and PS003), which clustered two strains each.
Figure 2.
Dendrograms obtained by clustering FTIR spectra for P. aeruginosa strains (n = 18). The vertical dashed lines represent the cut-off value. Green spectra indicate that the strain was a singleton, and orange spectra indicate a cluster formation. Different colors are used to differentiate the information regarding patient identification (A–G). Origin (COVID-19 or non-COVID-19 co-infection), antimicrobial susceptibility test (AST) antimicrobials that presented resistance (SXT: sulfamethoxazole-trimethoprim, MEM: meropenem, CAZ: ceftazidime, FOX: cefoxitin, CEF: cefepime, CRO: ceftriaxone, CXM: cefuroxime axetil, TZP: piperacillin-tazobactam, AMI: amikacin, GEN: gentamicin, CIP: ciprofloxacin, NOR: norfloxacin, IPM: imipenem), biofilm formation on polystyrene surface at 22.5 and 37 °C (- = non-adherent; + = weakly adherent; ++ = moderately adherent; and +++ = strongly adherent).
3.2. Evaluation of Antimicrobial Susceptibility Profile
The antimicrobial susceptibility profiles are presented in Figure 2 and Table 3. All the strains were resistant to CXM, CRO, and FOX but susceptible to AMI. According to EUCAST [3], the CRO resistance phenotype is already expected in P. aeruginosa strains, and this fact was observed in this study as predictable. Strain PS003 was the only strain resistant to NOR. Six (33.3%) strains were resistant to SXT, five (27.8%) were resistant to TZP, and eight (44.4%) were intermediate resistant. Eight (44.4%) strains showed intermediate resistance to CAZ and seven (38.9%) were resistant. Two (11.1%) strains were intermediate resistant to MEM, while four (22.2%) were resistant. Regarding the drug CIP, six (33.3%) strains were intermediate resistant and three (16.7%) were resistant. Twelve (66.6%) strains were classified as CEF intermediate resistance, and three (16.7%) were resistant. Seven (38.9%) strains were intermediate resistant to IPM and six (33.3%) were as resistant. The strains PS003 and PS006 were, respectively, resistant and intermediate resistant to GEN, while the remaining strains were susceptible. Fourteen (77.8%) strains (PS001, PS002, PS003, PS006, PS007, PS010, PS011, PS012, PS013, PS014, PS015, PS018, PS019 and PS020) were resistant to ≥5 antimicrobials tested and were classified as MDR according to Magiorakos et al. [17] (Table 3).
Table 3.
Antimicrobial susceptibility profile of P. aeruginosa strains (n = 18) by disk diffusion method.
| Strains | SXT | CAZ | CEF | CRO | CXM | FOX | TZP | AMI | GEN | CIP | NOR | MEM | IPM | Magiorakos et al. (2012) Classification [17] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS001.21 | R | I | I | R | R | R | I | S | S | S | S | S | I | MDR |
| PS002.21 | R | I | I | R | R | R | I | S | S | I | S | S | R | MDR |
| PS003.21 | S | R | R | R | R | R | R | S | R | R | R | R | R | MDR |
| PS005.21 | S | I | I | R | R | R | I | S | S | S | S | S | I | N-MDR |
| PS006.21 | S | R | R | R | R | R | R | S | I | I | S | R | R | MDR |
| PS007.21 | R | I | I | R | R | R | I | S | S | I | S | S | I | MDR |
| PS008.21 | S | S | S | R | R | R | S | S | S | S | S | S | S | N-MDR |
| PS010.22 | S | R | I | R | R | R | S | S | S | R | S | R | R | MDR |
| PS011.22 | S | R | R | R | R | R | R | S | S | S | S | S | S | MDR |
| PS012.22 | S | I | I | R | R | R | R | S | S | R | S | I | I | MDR |
| PS013.22 | R | I | I | R | R | R | I | S | S | I | S | S | I | MDR |
| PS014.22 | R | I | I | R | R | R | I | S | S | I | S | S | I | MDR |
| PS015.22 | S | R | I | R | R | R | R | S | S | S | S | S | S | MDR |
| PS016.22 | S | S | S | R | R | R | S | S | S | S | S | S | S | N-MDR |
| PS017.22 | S | S | S | R | R | R | S | S | S | S | S | S | S | N-MDR |
| PS018.22 | S | R | I | R | R | R | I | S | S | S | S | I | R | MDR |
| PS019.22 | S | R | I | R | R | R | S | S | S | S | S | R | R | MDR |
| PS020.22 | R | I | I | R | R | R | I | S | S | I | S | S | I | MDR |
R—resistant, I—intermediate resistant, S—sensitive, SXT—sulfamethoxazole-trimethoprim, MEM—meropenem, CAZ—ceftazidime, FOX—cefoxitin, CEF—cefepime, CRO—ceftriaxone, CXM—cefuroxime axetil, TZP—piperacillin-tazobactam, AMI—amikacin, GEN—gentamicin, CIP—ciprofloxacin, NOR—norfloxacin, IPM—imipenem, MDR—multidrug-resistant, N-MDR—no-multidrug-resistant.
Detailed information on the percentage of resistance found for each antimicrobial can be found in Supplementary Table S1.
3.3. Evaluation of Biofilm Formation and Its Tolerance to Disinfectants
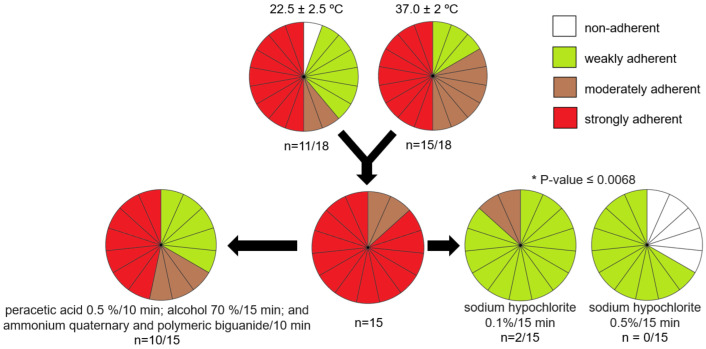
P. aeruginosa biofilm formation is presented in Figure 3. No significant statistical difference between the temperatures of incubation (22.5 ± 2.5 °C and 37 ± 2 °C) was observed (p = 0.18). At both temperatures, 50.0% (n = 9) of the strains formed strongly adherent biofilm. At 22.5 °C ± 2.5 °C, 11.1% (n = 2) of the strains were classified as moderate biofilm producers, 33.3% (n = 6) of strains produced weakly adherent biofilms and only one strain did not form biofilm. At 37 °C ± 2 °C, 33.3% (n = 6) of the strains were moderately adherent and 16,7% (n = 3) were weakly adherent.
Figure 3.
Biofilm formation of P. aeruginosa strains (n = 18) and assessment of biofilm sensitivity to disinfectants. Color legend: white: non-adherent; green: weak adherent; brown: moderate adherent; red: strong adherent. Significant p-values are presented.
Overall, 83.3% (n = 15) of the strains were classified as moderately or strongly adherent and were submitted to the biofilm disinfectant tolerance test. Exposure to peracetic acid (0.5%), alcohol (70%), and ammonium quaternary fifth-generation/stabilized polymeric biguanide did not reduce biofilm formation (p ≥ 0.0832). Significant statistical difference in biofilm formation was observed only for sodium hypochlorite (0.1% and 0.5%) (p = 0.0068); nevertheless, only the higher concentration (0.5%) reduced non-adherence in 22.2% (n = 4) of the strains (Figure 3).
Detailed information on biofilm formation and its tolerance to disinfectants can be found in Supplementary Tables S2 and S3.
4. Discussion
P. aeruginosa is an opportunistic pathogen that is often related to nosocomial infections, especially in intensive care units (ICUs). It has been responsible for several hospital outbreaks around the world [1]; therefore, its accurate and rapid identification is warranted. Furthermore, accurate bacterial identification is very important for bacterial epidemiology and for the appropriate use of antimicrobials [20]. In the present study, the 16S rRNA gene sequencing was not able to distinguish between P. aeruginosa and P. paraeruginosa, indicating that these two species have a very close genotypic relationship. These two species aligned together in the phylogenetic tree, showing that this gene is highly conserved between them (Figure 1). Similar results were observed in the study of Rudra et al. [21], which also verified that the 16S rRNA gene sequencing is not a reliable method for P. aeruginosa and P. paraeruginosa differentiation.
The MALDI-TOF/MS (using both systems) was able to identify all the P. aeruginosa strains to the species level (Table 1). MALDI-TOF/MS is a technology used worldwide due to its high accuracy and rapidness while also being cost-effective for bacterial identification [22]. According to Mulet et al. [23], the MALDI-TOF MS proved to maintain high sensitivity and specificity for the early identification of high-risk clones of P. aeruginosa in several clinical isolates. Wamer et al. [24] also reported that MALDI-TOF MS is an effective technique for the identification of P. aeruginosa.
In the FTIR analysis, the IR 11 profile clustered four strains together which were able to form a moderate or strong biofilm. Three strains (PS010.22, PS012.22, and PS013.22) were isolated from the same location (ICU-2), and two (PS010.22 and PS012.22) of them were isolated during HIPCC monitoring. In addition, biofilms formed by PS010.22 and PS013.22 were tolerant to all disinfectants tested, except for sodium hypochlorite. These results suggest that biofilm formation could have been an important factor in the persistence of this cluster in the hospital. The IR 8 profile clustered two strains (PS005.21 and PS011.21) together. These two strains formed strong biofilms which were tolerant to all disinfectants tested, except for sodium hypochlorite. In the IR 13 profile cluster, which grouped the PS002.21 and PS003.21, it was noted that both strains were isolated from patients with COVID-19 in the ICU-1, and produced strong biofilms which were tolerant to all disinfectants tested, except for sodium hypochlorite. The strains were resistant to ≥5 drugs tested, and 77.78% of the strains were classified as MDR.
Therefore, FTIR was shown to be a useful tool for P. aeruginosa typing. In the study of Martak et al. [5], this technique was reported to be a very useful approach for differentiating P. aeruginosa clones responsible for hospital outbreaks. These results also corroborate with the previous study where FTIR was used for the surveillance of another nosocomial Gram-negative bacilli, Stenotrophomonas maltophilia, where the methodology was able to differentiate strains from clinical and environmental sources [25].
The therapeutic options for P. aeruginosa infections are restricted due to its intrinsic and adaptative resistance to antibiotics commonly prescribed [3]. Furthermore, studies indicate an increase of up to 50% in the emergence of MDR P. aeruginosa strains during the COVID-19 pandemic, which made therapeutic management even more limited and increased patient mortality [26]. In the present study, all the strains were resistant to cefuroxime axetil, ceftriaxone, and cefoxitin, and 77.78% of the strains were classified as MDR.
For a long time, β-Lactam antibiotics have been a mainstay in the treatment of P. aeruginosa; however, several β-Lactam-resistant strains have been reported, which has restricted even more the therapeutic options available [27]. In the present study, the highest rate of resistance was observed in β-Lactam antibiotics, where 83.3% (n = 15) of the strains showed resistance or intermediate resistance to ceftazidime, followed by cefepime with 77.8% (n = 14) and piperacillin-tazobactam with 72.2% (n = 13).
Due to the high emergence of carbapenem-resistant P. aeruginosa (CRPA) worldwide, the World Health Organization (WHO) [28] has included CRPA in the list of pathogens of high priority for the research and development of new antibiotics [29]. In the present study, 72.2% (n = 13) of the strains were resistant or intermediate resistant to imipenem and 33.3% (n = 6) were resistant or intermediate resistant to meropenem.
The sulfonamides are considered an alternative for the treatment of P. aeruginosa infections resistant to carbapenems [30]. However, due to the ability of P. aeruginosa to acquire new resistance via mutation and horizontal gene transfer, resistant strains are being reported [31]. In the present study, 33.3% of the strains were resistant to sulfamethoxazole-trimethoprim. Similar results were observed in the study of Haghi et al. [31], where 77.4% of the P. aeruginosa were sulfamethoxazole-trimethoprim resistant.
The aminoglycosides showed favorable activity against P. aeruginosa strains since all the strains were susceptible to amikacin and only the strains PS003.21 and PS006.21 were, respectively, resistant and intermediate resistant to gentamicin. Moreover, the fluoroquinolones-class antibiotic, norfloxacin, can also be considered promising treatment options against P. aeruginosa; only the strain PS003.21 was resistant. In contrast, 50.0% (n = 9) of the strains were resistant or intermediate resistant to ciprofloxacin. Shiralizadeh et al. [32] also reported an increase in fluoroquinolone resistance rates, such as ciprofloxacin, where 60% of the P. aeruginosa were resistant.
The incidence of infections caused by P. aeruginosa increased significantly during the COVID-19 pandemic, especially due to its ability to form biofilm in medical devices such as catheters, nebulizers, and humidifiers [9,10,26,33]. In the present study, all the strains were able to adhere and produce biofilms in polystyrene surfaces at 37 °C ± 2 °C and only the strain PS014.22 did not produce biofilms at 22.5 °C ± 2.5 °C. Overall, 83.3% (n = 15) were characterized as moderately or strongly adherent (Figure 3). These data corroborate the study of Patel and Gajjar [34], where 16 P. aeruginosa strains were strong or moderate biofilm producers.
The biofilm formation contributes not only to the adherence to insert surfaces but can also mediate resistance to several antibiotics and tolerance to disinfectants, hence contributing to the emergence of MDR strains and making the selection of an appropriate cleaning procedure limited [4,35]. In the disinfectant tolerance test, only sodium hypochlorite was able to reduce the biofilm formation in P. aeruginosa, reducing 22.2% of the strains to non-adherence at a concentration of 0.5%. Nevertheless, it cannot be used on corrosion-sensitive surfaces, and, therefore, alternative sanitizers/disinfectants and contact periods need to be considered.
5. Conclusions
In conclusion, VITEK MS® and MALDI Biotyper® correctly identified P. aeruginosa strains. The 16S rRNA gene sequencing did not differentiate between the species P. aeruginosa and P. paraeruginosa. FTIR was considered a useful tool for P. aeruginosa typing. Most of the strains were resistant or intermediate resistant to the drugs tested. In particular, AMI and NOR were demonstrated to be the best treatment options for P. aeruginosa, since almost all the strains were susceptible to these two antimicrobials. Fourteen (77.8%) strains were classified as MDR. Almost all the strains produced biofilms on polystyrene surfaces, and sodium hypochlorite (0.5%) was the most effective disinfectant for its elimination. Nevertheless, it is important that further cleaning protocols are evaluated to eradicate this biofilm-producing organism from different surface materials, and consequently reduce the incidence of infection by P. aeruginosa. The small number of strains returned from the hospital is a notable limitation of the study, however, several complementary methodologies were applied aiming to give robustness to the present research.
Acknowledgments
The authors are grateful to Bio-Manguinhos/Fiocruz, the Postgraduation Program in Biodiversity and Health Program of IOC/Fiocruz, CNPq, CAPES, and FAPERJ.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life14091079/s1, Table S1: Percentages of resistance, intermediary resistance and susceptibility of the P. aeruginosa strains (n = 18) to each antibiotic tested; Table S2: Biofilm formation of P. aeruginosa strains (n = 18); Table S3: Assessment of P. aeruginosa strain (n = 15) biofilm sensitivity to disinfectants.
Author Contributions
Conceptualization, P.A.d.S., L.V.d.C., M.L.L.B., R.V.d.S.L.d.M. and M.H.S.V.B.; methodology, P.A.d.S., M.C.S.d.S., R.V.d.S.L.d.M., L.V.d.C., R.P.P.d.S., C.A.C.d.M. and M.L.L.B.; formal analysis, P.A.d.S., M.C.S.d.S., A.P.R.d.S., S.J.F., C.A.C.d.M., M.H.S.V.B. and M.L.L.B.; investigation, P.A.d.S., M.C.S.d.S., R.V.d.S.L.d.M., L.V.d.C., R.V.d.S.L.d.M., C.A.C.d.M. and M.L.L.B.; resources, M.H.S.V.B. and M.L.L.B.; data curation, P.A.d.S., M.C.S.d.S., R.V.d.S.L.d.M., L.V.d.C., R.P.P.d.S., C.A.C.d.M. and M.L.L.B.; writing—original draft preparation, P.A.d.S.; writing—review and editing, L.V.d.C., R.P.P.d.S., C.A.C.d.M., S.J.F., M.H.S.V.B. and M.L.L.B.; visualization, P.A.d.S., M.C.S.d.S., A.P.R.d.S., S.J.F., M.H.S.V.B. and M.L.L.B.; supervision, M.L.L.B. and M.H.S.V.B.; project administration, M.L.L.B. and M.H.S.V.B.; funding acquisition, M.L.L.B. and M.H.S.V.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Hospital de Força Aérea do Galeão (HFAG) Ethics and Research Committee and is registered on Plataforma Brasil with CAAE code: 55303721.0.0000.5250.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article are available in the article.
Conflicts of Interest
Author Prof SJ Forsythe was employed by the company Foodmicrobe.com Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Funding Statement
This research was funded in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq): CNPq/MCTI/FNDCT N° 18/2021-Faixa A-Grupos Emergentes Processo n.°407747/2021-4, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ)–PROGRAMA E_13/2023–AUXÍLIO BÁSICO À PESQUISA (APQ1)–2023-Ref. Proc. E-26/210.616/2024.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pulusu C.P., Manivannan B., Raman S.S., Singh S., Khamari B., Lama M., Peketi A.S.K., Datta C., Prasad K.N., Nagaraja V., et al. Localized outbreaks of Pseudomonas aeruginosa belonging to international high-risk clones in a south Indian hospital. J. Med. Microbiol. 2022;71:29–40. doi: 10.1099/jmm.0.001500. [DOI] [PubMed] [Google Scholar]
- 2.Albin O.R., Kaye K.S., McCreary E.K., Pogue J.M. Less Is More? Antibiotic Treatment Duration in Pseudomonas aeruginosa Ventilator-Associated Pneumonia. Clin. Infect. Dis. 2023;76:745–749. doi: 10.1093/cid/ciac784. [DOI] [PubMed] [Google Scholar]
- 3.EUCAST . European Committee on Antimicrobial Susceptibility Testing (EUCAST). Expected Resistant and Susceptible Phenotypes. EUCAST; Copenhagen, Denmark: 2021. [Google Scholar]
- 4.Pang Z., Raudonis R., Glick B.R., Lin T.J., Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Martak D., Valot B., Sauget M., Cholley P., Thouverez M., Bertrand X., Hocquet D. Fourier-Transform InfraRed Spectroscopy can quickly type Gram-negative bacilli responsible for hospital outbreaks. Front. Microbiol. 2019;10:1440. doi: 10.3389/fmicb.2019.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrero-Lozano I., Galán-Sánchez F., Rodríguez-Iglesias M. Fourier transform infrared spectroscopy as a new tool for surveillance in local stewardship antimicrobial program: A retrospective study in a nosocomial Acinetobacter baumannii outbreak. Braz. J. Microbiol. 2022;53:1349–1353. doi: 10.1007/s42770-022-00774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muchaamba F., Stephan F. A Comprehensive Methodology for Microbial Strain Typing Using Fourier-Transform Infrared Spectroscopy. Methods Protoc. 2024;7:48. doi: 10.3390/mps7030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oho M., Nagasawa Z., Funashima Y., Ueda O., Watamabe S., Cui L., Miyamoto H., Sueoka E. Correlation of Strain Classification with IR Biotyper and Molecular Epidemiological Method of Pseudomonas aeruginosa. J. Assoc. Rapid Method Autom. Microbiol. 2021;31:29–40. [PubMed] [Google Scholar]
- 9.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu X.J., Ge Y., Wu T., Zhao K., Chen Y., Wu B., Zhu F., Zhu B., Cui L. Coinfection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285:198005. doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pericolini E., Colombari B., Ferretti G., Iseppi R., Ardizzoni A., Girardis M., Sala A., Peppoloni S., Blasi E. Real-time monitoring of Pseudomonas aeruginosa biofilm formation on endotracheal tubes in vitro. BMC Microbiol. 2018;18:84. doi: 10.1186/s12866-018-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaspari R., Spinazzola G., Teofili L., Avolio A.W., Fiori B., Maresca G.M., Spanu T., Nicolotti N., De Pascale G., Antonelli M. Protective effect of SARS-CoV-2 preventive measures against ESKAPE and Escherichia coli infections. Eur. J. Clin. Investig. 2021;51:e13687-94. doi: 10.1111/eci.13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu J., Cai Z., Liu Y., Duan X., Han S., Liu J., Zhu Y., Jiang Z., Zhang Y., Zhuo C., et al. Persistent bacterial coinfection of a COVID-19 patient caused by a genetically adapted Pseudomonas aeruginosa chronic colonizer. Front. Cell. Infect. Microbiol. 2021;11:641920. doi: 10.3389/fcimb.2021.641920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon S.H., Ha S.-M., Kwon S., Lim J., Kim Y., Seo H., Chun J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura K. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100; Wayne, PA, USA: 2020. [Google Scholar]
- 17.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 18.Vasconcellos L., Silva S.V., da Costa L.V., da Silva Lage de Miranda R.V., Reis C.M.F.D., da Silva Braga L.M.P., Silva C., Conceição G., Mattoso J., Silva I.B., et al. Phenotypical and molecular characterization of Acinetobacter spp. isolated from a pharmaceutical facility. Lett. Appl. Microbiol. 2023;76:101. doi: 10.1093/lambio/ovad101. [DOI] [PubMed] [Google Scholar]
- 19.Stepanović S., Vuković D., Hola V., Bonaventura G.D., Djukić S., Ćirković I., Ruzicka F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci,” APMIS: Acta pathologica, microbiologica, et immunologica Scandinavica. APMIS. 2007;115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 20.Chuang Y.C., Sheng W.H., Li S.Y., Lin Y.C., Wang J.T., Chen Y.C., Chang S.C. Influence of genospecies of Acinetobacter baumannii complex on clinical outcomes of patients with Acinetobacter bacteremia. Clin. Infect. Dis. 2011;52:352–360. doi: 10.1093/cid/ciq154. [DOI] [PubMed] [Google Scholar]
- 21.Rudra B., Duncan L., Shah A.J., Shah H.N., Gupta R.S. Phylogenomic and comparative genomic studies robustly demarcate two distinct clades of Pseudomonas aeruginosa strains: Proposal to transfer the strains from an outlier clade to a novel species Pseudomonas paraeruginosa sp. nov. Int. J. Syst. Evol. Microbiol. 2022;72:11–24. doi: 10.1099/ijsem.0.005542. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchida S., Umemura H., Nakayama T. Current Status of Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight Mass Spectrometry (MALDI-TOF MS) in Clinical Diagnostic Microbiology. Molecules. 2020;25:4775. doi: 10.3390/molecules25204775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulet X., Fernández-Esgueva M., Norte C., Zamorano L., del Barrio-Tofiño E., Oliver A., GEMARA-SEIMC-REIPI Pseudomonas study group Validation of MALDI-TOF for the early detection of the ST175 high-risk clone of Pseudomonas aeruginosa in clinical isolates belonging to a Spanish nationwide multicenter study. Enferm. Infecc. Microbiol. Clin. 2021;39:279–282. doi: 10.1016/j.eimce.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Wamer C.N., Morse C.N., Gadient J.N., Dodson T.A., Carlson E.A., Prestwich E.G. Comparison of Small Biomolecule Ionization and Fragmentation in Pseudomonas aeruginosa Using Common MALDI Matrices. J. Am. Soc. Mass. Spectrom. 2023;34:355–365. doi: 10.1021/jasms.2c00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souza P.A., Miranda R.V.S.L., Santos M.C.S., Costa L.V., Silva R.P.P.S., Miranda C.A.C., Bôas M.H.S., Brandão M.L.L. Evaluation of Fourier-Transform Infrared Spectroscopy as a rapid method to type Stenotrophomonas maltophilia strains isolated from pharmaceutical industry; Proceedings of the 8th International Symposium on Immunobiologicals; Rio de Janeiro, Brazil. 8–10 May 2024; [(accessed on 15 August 2024)]. Available online: https://www.arca.fiocruz.br/handle/icict/63778. [Google Scholar]
- 26.Ng Q.X., Ong N.Y., Lee DY X., Yau C.E., Lim Y.L., Kwa AL H., Tan B.H. Trends in Pseudomonas aeruginosa (P. aeruginosa) bacteremia during the COVID-19 pandemic: A systematic review. Antibiotics. 2023;12:409. doi: 10.3390/antibiotics12020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Idowu T., Ammeter D., Brizuela M., Jackson G., Alam S., Schweizer F. Overcoming β-Lactam resistance in Pseudomonas aeruginosa using non-canonical tobramycin-based antibiotic adjuvants. Bioorg. Med. Chem. Lett. 2020;30:127575. doi: 10.1016/j.bmcl.2020.127575. [DOI] [PubMed] [Google Scholar]
- 28.WHO . WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. WHO; Geneva, Switzerland: 2024. [Google Scholar]
- 29.Halat D.H. Moubareck CA, The Intriguing Carbapenemases of Pseudomonas aeruginosa: Current Status, Genetic Profile, and Global Epidemiology. Yale J. Biol. Med. 2022;95:507–515. [PMC free article] [PubMed] [Google Scholar]
- 30.Mizdal C.R. Molecular docking, and anti-biofilm activity of gold-complexed sulfonamides on Pseudomonas aeruginosa. Microb. Pathog. 2018;125:393–400. doi: 10.1016/j.micpath.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Haghi F., Zeighami H., Monazami A., Toutouchi F., Nazaralian S., Naderi G. Diversity of virulence genes in multidrug resistant Pseudomonas aeruginosa isolated from burn wound infections. Microb. Pathog. 2018;115:251–256. doi: 10.1016/j.micpath.2017.12.052. [DOI] [PubMed] [Google Scholar]
- 32.Shiralizadeh S., Keramat F., Hashemi S.H., Majzoobi M.M., Azimzadeh M., Alikhani M.S., Karami P., Rahimi Z. Investigation of antimicrobial resistance patterns and molecular typing of Pseudomonas aeruginosa isolates among Coronavirus disease-19 patients. BMC Microbiol. 2023;23:84. doi: 10.1186/s12866-023-02825-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassetti M., Vena A., Croxatto A., Righi E., Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Cont. 2018;7:212527. doi: 10.7573/dic.212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel H., Gajjar D. Cell adhesion and twitching motility influence strong biofilm formation in Pseudomonas aeruginosa. Biofouling. 2022;38:235–249. doi: 10.1080/08927014.2022.2054703. [DOI] [PubMed] [Google Scholar]
- 35.Karash S., Yahr T.L. Genome-Wide Identification of Pseudomonas aeruginosa Genes Important for Desiccation Tolerance on Inanimate Surfaces. mSystems. 2022;7:e0011422. doi: 10.1128/msystems.00114-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article.