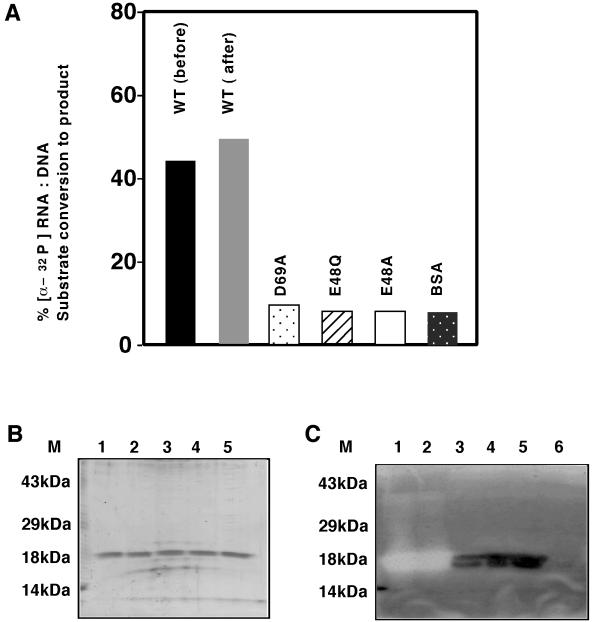
FIG. 2.
RNase H assays of purified recombinant proteins. Mo-MuLVrnh157 and mutant E48A, E48Q, and D69A proteins were expressed and purified as histidine fusions from MIC3001 transformants. All fractions analyzed corresponded to the 100 mM imidazole eluate of nickel chelate agarose columns. (A) RNase H solution assay. Aliquots (2.8 μg) of proteins were assayed at 37°C for activity against [α-32P]poly(rA):(dT)5500 heteroduplex in the presence of 1.0 mM MnCl2. Conversion of substrate to product is indicated as percent conversion. Abbreviations: WT (before), wild type Mo-MuLVrnh157 protein expressed and purified prior to four rounds of complementation assay; WT (after), wild type MoMuLVrnh157 protein expressed and purified after to four rounds of complementation assay in MIC3001; BSA, bovine serum albumin. (B) Coomassie blue-stained gel of purified recombinant proteins used in panel A (350 ng of protein per lane). Lane 6, 100 mM nickel chelate agarose eluate of extracts prepared from p6His-3-transformed MIC3001. (C) In situ RNase H gel renaturation assays of protein shown in panel B. (B and C) Lanes: M, prestained molecular weight markers (Gibco-BRL) 1, Mo-MuLVrnh157 before complementation; 2, Mo-MuLVrnh157 after complementation; 3, D69A mutant protein; 4, E48Q mutant protein; 5, E48A mutant protein. Renaturation assays were performed in 1 mM MnCl2.