Figure 8.
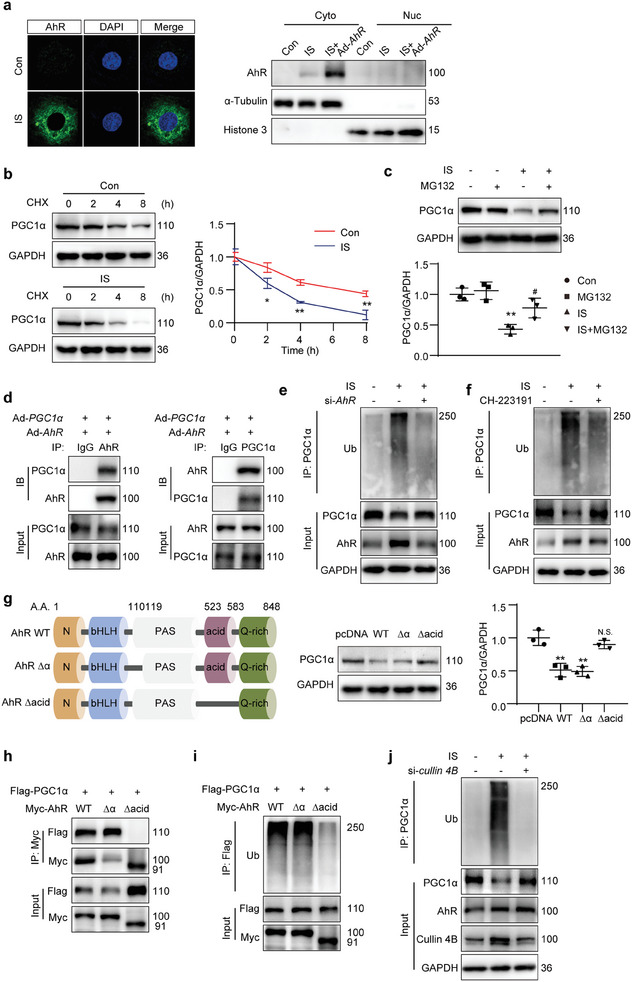
AhR promoted PGC1α degradation via ubiquitin modification. a) Immunofluorescence micrographs and Western blot presenting AhR distribution in mTECs after IS treatment (1000 µmol L−1) for 12 h, with or without transfection using adenovirus carrying AhR gene (Ad‐AhR) for 36 h. b) Western blot images and quantitative data detecting the PGC1α in mTECs with IS treatment (1000 µmol L−1) for 36 h. Cells were treated with cycloheximide (CHX, 10 µmol L−1) for the indicated durations before being harvested. * P < 0.05, ** P < 0.01 versus Con group (n = 3). c) Western blot images and quantitative data detecting the PGC1α in mTECs subjected to 36‐h IS treatment (1000 µmol L−1) in the presence of MG132 (20 µmol L−1) for 4 h. ** P < 0.01 versus Con group, # P < 0.05 versus IS group (n = 3). d) Co‐IP of AhR and PGC1α in mTECs cotransfected with Ad‐AhR and Ad‐PGC1α with IS stimulation (1000 µmol L−1) for 48 h. The lysates were immunoprecipitated with control IgG, anti‐AhR, or anti‐PGC1α antibodies, followed by Western blot with the indicated antibody (n = 3). e,f) IP analyzing the ubiquitination of PGC1α in mTECs. The cells were transfected with AhR siRNA (si‐AhR; e) or treated with AhR inhibitor CH‐223191 (10 µmol L−1; f) in the presence of IS (1000 µmol L−1) for 48 h. MG132 (20 µmol L−1) was added to the medium 4 h before cell harvest. The lysates were immunoprecipitated with anti‐PGC1α antibody, followed by Western blot with anti‐ubiquitin (Ub) antibody (n = 3). g) Schematic of full‐length and truncated AhR mutants. Western blot images and quantitative data detecting the PGC1α in mTECs. The cells were transfected with Myc‐tagged pcDNA, AhR full‐length (WT), 110–119 amino acid‐deletion mutant (Δα), or acidic domain‐deletion mutant (Δacid) vectors with IS stimulation (1000 µmol L−1) for 48 h. ** P < 0.01, N.S. (no significant difference) versus pcDNA group (n = 3). h) Co‐IP of AhR or mutants with PGC1α in HEK293T cells cotransfected with plasmids encoding Flag‐tagged PGC1α and Myc‐tagged AhR WT, AhR Δα or AhR Δacid with IS stimulation (1000 µmol L−1) for 48 h. The lysates were immunoprecipitated with anti‐Myc antibody, followed by Western blot with anti‐Flag antibody (n = 3). i) IP of AhR or mutants with PGC1α in HEK293T cells cotransfected with plasmids encoding Flag‐tagged PGC1α and Myc‐tagged AhR WT, AhR Δα or AhR Δacid in the presence of IS (1000 µmol L−1) for 48 h. MG132 (20 µmol L−1) was added to the medium 4 h before cell harvest. The lysates were immunoprecipitated with anti‐Flag antibody, followed by Western blot with anti‐Ub antibody (n = 3). j) IP analysis of PGC1α ubiquitination in mTECs transfected with cullin 4B siRNA (si‐cullin 4B) in the presence of IS (1000 µmol L−1) for 48 h. MG132 (20 µmol L−1) was added to the medium 4 h before cell harvest. The lysates were immunoprecipitated with anti‐PGC1α antibody, followed by Western blot with anti‐ubiquitin (Ub) antibody (n = 3). Data were shown as mean ± SD. Statistical analysis was performed by two‐way ANOVA with Tukey's multiple comparisons test (b, c, g).
