FIG. 1.
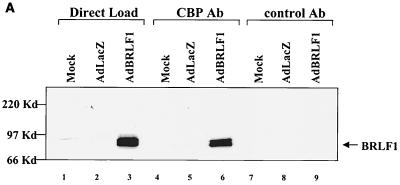
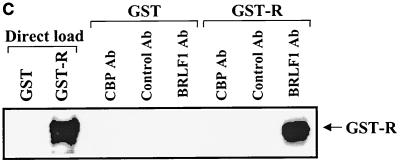
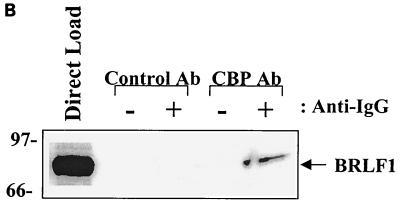
BRLF1 interacts with CBP in vivo. (A) HeLa cells were mock infected or infected with adenovirus vectors expressing beta-galactosidase (AdLacZ) or BRLF1 (AdBRLF1). Anti-CBP antibody (Ab) or a control rabbit antibody were used to coimmunoprecipitate BRLF1 (300 μg; lanes 4 to 9). Immunocomplexes were electrophoresed on a 7.5% polyacrylamide gel, transferred to nitrocellulose, and immunoblotted for BRLF1. Proteins were visualized by chemiluminescence and autoradiography. Direct loads (30 μg; lanes 1 to 3) confirmed the presence of BRLF1 in the HeLa cell extracts. (B) Coimmunoprecipitation experiments (as described for panel A) were performed using extracts from Akata cells with or without anti-immunoglobulin G (anti-IgG) treatment (100 μg/ml; Sigma) for 4 h, which induces lytic EBV infection. The direct load lane is lysate from Akata cells treated with an anti-IgG antibody. (C) The same CBP and control rabbit antibodies used for panels A and B as well as a BRLF1-reactive antibody were used to immunoprecipitate GST or GST-BRLF1 fusion protein (GST-R), followed by immunoblot analysis with a BRLF1 antibody.