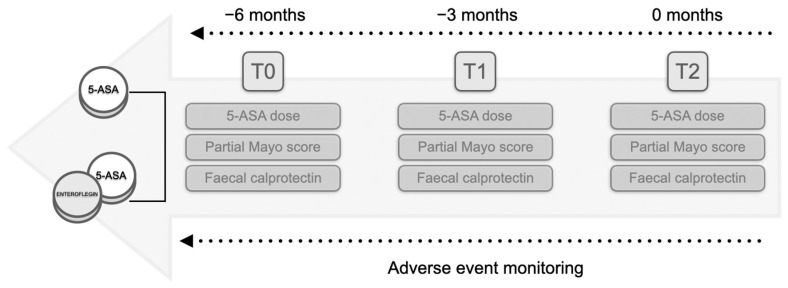
Figure 1.
Flowchart of the study protocol. The study comprised two groups: patients undergoing therapy solely with derivatives of 5-aminosalicylic acid (5-ASA) and patients receiving add-on nutraceutical supplementation. Retrospective analysis involved the collection of the partial Mayo score alongside the faecal calprotectin levels and the 5-ASA dose at three months (T1) and six months (T2) from the initiation of treatment at T0 (a retrospective window of six months). Throughout the interval, any reported adverse events were sought in the records.