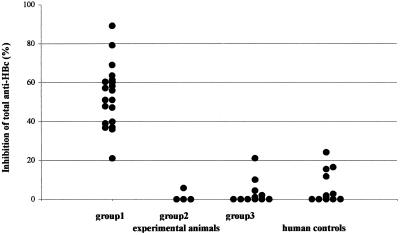
FIG. 2.
HBcAg specificity of antibodies induced by intrasplenic transfer of hu-PBL from healthy volunteers in the presence of HBcAg. The capacity to inhibit the binding of labeled human anti-HBc antibodies present in the ETI-AB-COREK-2 assay (DiaSorin) by plasma derived from mice in different experimental groups and human controls was measured. Group 1 consisted of NOD/SCID mice inoculated on day 0 with hu-PBL and rHBcAg; group 2 consisted of NOD/SCID mice given hu-PBL and rHBeAg on day 0, and group 3 contained mice given hu-PBL in PBS. All mouse plasma samples were obtained 14 days after the transfer of hu-PBL (with or without antigen). Twelve plasma samples from healthy blood donors without previous exposure to HBV were tested as well. The degree of inhibition of binding of labeled anti-HBc antibody present in the kit by the different mouse and human plasma samples is displayed.