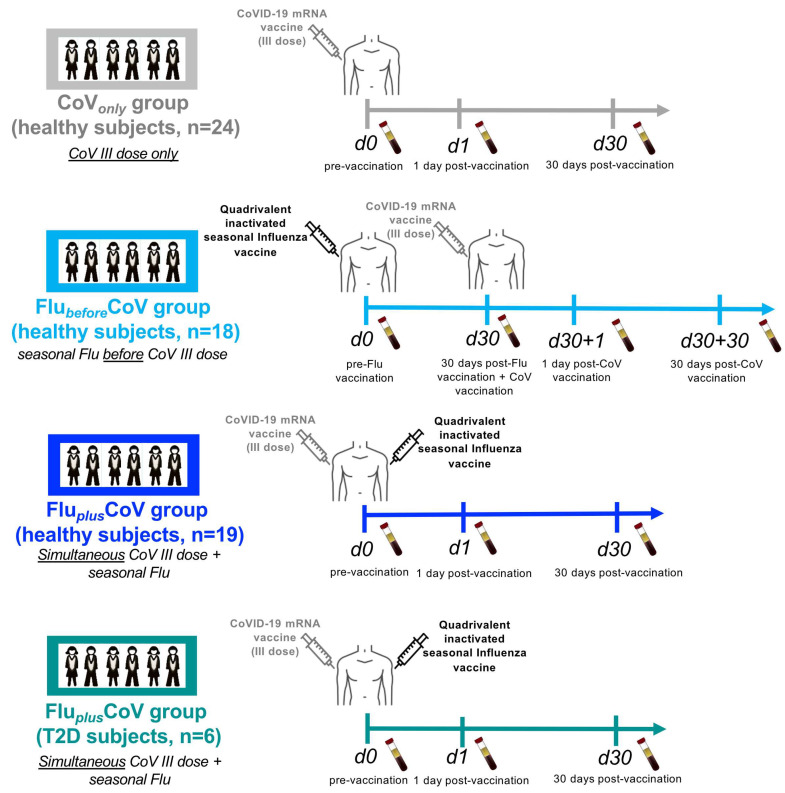
Figure 1.
Scheme of enrollment and collected samples from healthy and type 2 diabetes mellitus (T2D) vaccine recipients. A schematic representation of groups, number of study participants and timeline of sample collection are depicted. “CoVonly” group (in grey) comprised of healthy subjects (HS) (n = 24) to whom only the third booster dose of anti-COVID-19 BNT162b2 mRNA vaccine was administered. “FlubeforeCoV” group comprised of HS (n = 18) (in light blue) to whom the 2021–2022 seasonal cell-based quadrivalent influenza (Flu) vaccine (Flucelvax) was administered 1 month before immunization with the third booster dose of anti-COVID-19 BNT162b2 mRNA vaccine. “FluplusCoV” groups comprised of HS (n = 19) (in blue) and of T2D (n = 6) (in petrol green), to whom 2021–2022 seasonal cell-based quadrivalent flu vaccine (Flucelvax) was simultaneously inoculated in different limbs with the third booster dose of anti-COVID-19 BNT162b2 mRNA vaccine. Demographic and clinical information as well as serum samples from longitudinal peripheral blood withdrawals were collected at the following time points: immediately before (day 0, d0), as well as 1 day and 30 days (d1 and d30, respectively) after the third booster dose of COVID-19 mRNA vaccine in the presence or absence of the flu vaccine. Only for “FlubeforeCoV” group sera were stored immediately before (day 0, d0) quadrivalent flu vaccine, as well as before (day 30, d30) and 1 and 30 days after (d30 + 1 and d30 + 30, respectively) the third booster dose of the COVID-19 mRNA vaccine.