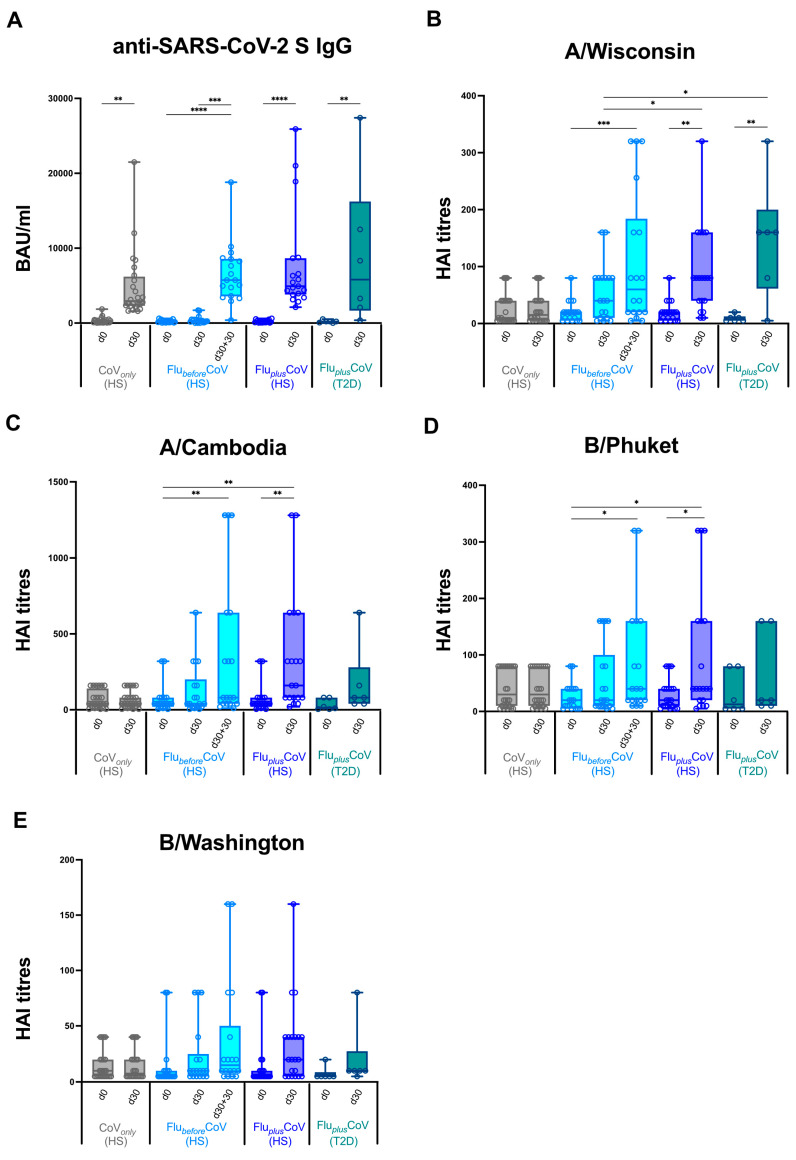
Figure 2.
Anti-influenza and anti-COVID-19 vaccine-specific antibody production in healthy and type 2 diabetes mellitus (T2D) vaccine recipients. Levels of binding class G immunoglobulins recognizing SARS-CoV-2 trimeric spike protein (anti-SARS-CoV-2 S IgG; expressed as BAU/mL) (A) and specific anti-influenza (Flu) antibodies against all the vaccine antigen components included in the Flucelvax Quadrivalent flu vaccine used in the 2021–2022 season (A/Wisconsin, A/Cambodia, B/Phuket and B/Washington; expressed as hemagglutination inhibition, HAI, titers) (B–E) were measured in “CoVonly” group (in grey), “FlubeforeCoV” (in light blue) as well as “FluplusCoV” groups (in blue for healthy subjects, HS; in petrol green for T2D individuals). Serum samples were analyzed before (day 0, d0) and 30 days (d30) after the third booster dose of COVID-19 mRNA vaccine in the presence or absence of flu vaccination. Only for “FlubeforeCoV” subjects, sera were tested before (day 0, d0) quadrivalent flu vaccine, as well before (day 30, d30) and 30 days after the third booster dose of COVID-19 mRNA vaccine (d30 + 30). p-values calculated by one-way ANOVA test were assigned as follows: * ≤ 0.05; ** ≤ 0.01; *** ≤ 0.001, **** ≤ 0.0001.