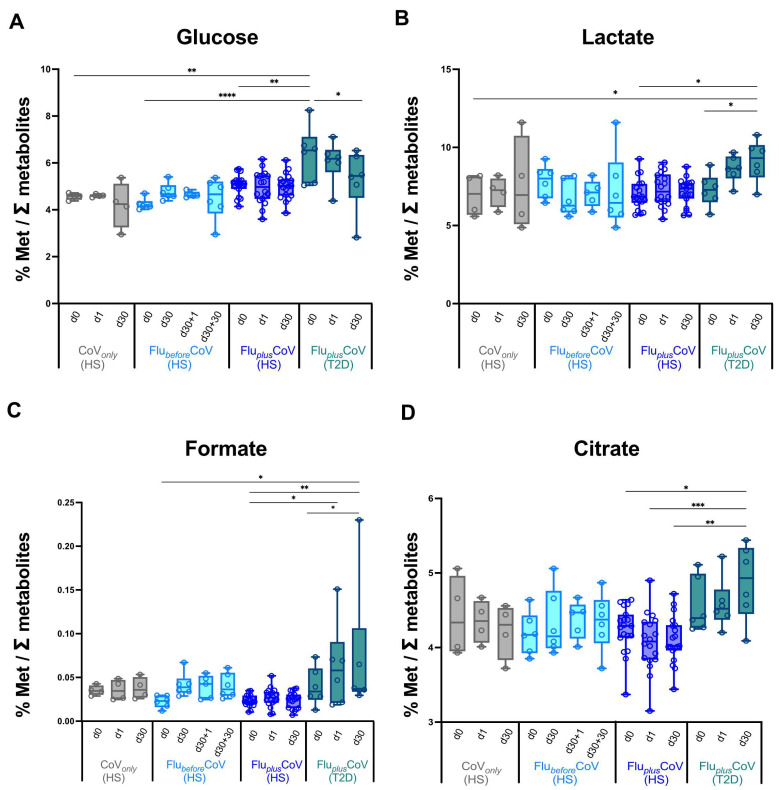
Figure 5.
Regulation of circulating metabolites involved in glucose/pyruvate pathways in healthy and type 2 diabetes mellitus (T2D) individuals receiving influenza and COVID-19 vaccination. Changes in glucose (A), lactate (B), formate (C) and citrate (D) levels were measured in serum samples longitudinally collected in fasting condition from the “CoVonly” group (in grey) and “FluplusCoV” groups (in blue for healthy subjects, HS; in petrol green for T2D individuals) immediately before (day 0, d0), as well as 1 and 30 days (d1 and d30, respectively) after the third booster dose of the COVID-19 mRNA vaccine in presence or absence of the flu vaccine. Only for the “FlubeforeCoV” group (in light blue) was the analysis performed on sera stored before (day 0, d0) the quadrivalent flu vaccine, as well as before (day 30, d30) and 1 and 30 days after (d30 + 1 and d30 + 30, respectively) the third booster dose of COVID-19 mRNA vaccine. Values are shown as percentage (%) of the metabolite level relative to total metabolites. p-values calculated by one-way ANOVA test were assigned as follows: * ≤ 0.05; ** ≤ 0.01; *** ≤ 0.001, **** ≤ 0.0001.