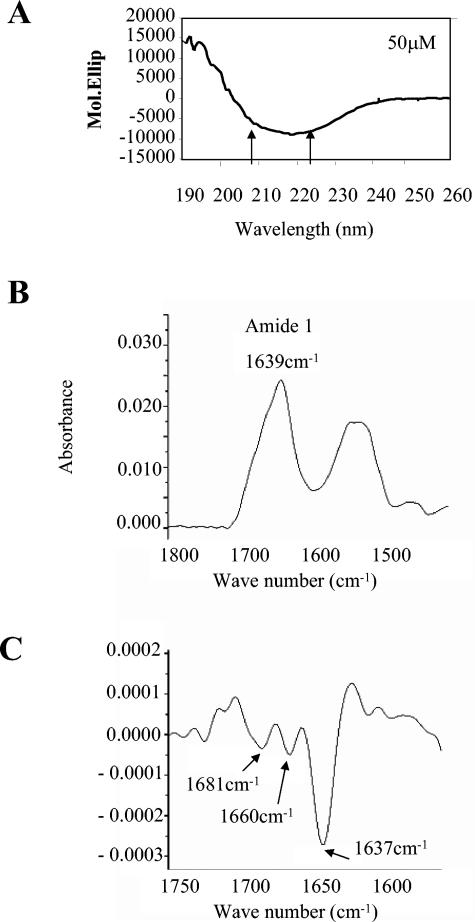
FIG. 4.
The proposed leucine zipper region of K-bZIP does not fold as a coiled coil in vitro. A synthetic peptide corresponding to amino acids 182 to 218 of K-bZIP was synthesized and analyzed by circular dichroism spectroscopy as described in references 15 and 49. A. The normalized spectrum of a 50 μM peptide solution at pH 3.7 is shown. The arrows indicate the two minima, which are characteristic of α-helical secondary structure. The observed minimum, 218 nm, is characteristic of a β-sheet. B. The FTIR-ATR spectrum of peptide is shown. The position of the amide I peak is indicated. C. The second derivative spectrum of the amide I peak revealed three distinct contributions at the indicated wavelengths. The characteristic structure responsible for generating a peak at that wavelength is shown.