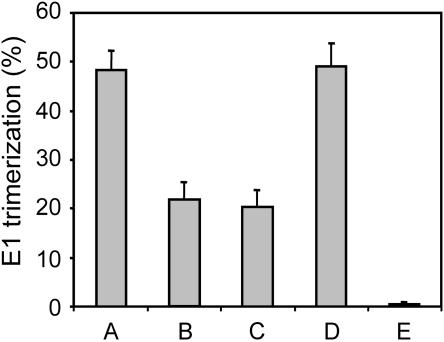
FIG. 3.
Effects of acidification, neutralization, and reacidification on E1 trimerization. Trimerization of E1 was determined under the same experimental conditions as in the fusion and binding experiments. Radioactively labeled SFV (105 to 106 cpm) in HNE buffer in a glass tube was continuously stirred at 37°C. Bar A represents SFV that was acidified to pH 5.5 by the injection of a small, pretitrated volume of 0.1 M MES and 0.2 M acetic acid in the presence of PC-PE-Chol-SPM (1:1:1.5:1) LUVs. After 5 min, the mixture was neutralized by the addition of a small amount of 0.1 M NaOH and placed on ice. Bar B represents SFV alone that was acidified and, 5 min later, neutralized and placed on ice. Bar C represents SFV alone that was acidified and to which, at 2 min, PC-PE-Chol-SPM LUVs were added. At 5 min, the mixture was neutralized and placed on ice. Bar D represents SFV alone that was acidified and for which, 5 min later, the pH of the mixture was raised to pH 8. Subsequently, the LUVs were added, and the mixture was reacidified to pH 5.5. After 1 min, the mixture was neutralized and placed on ice. Bar E represents SFV alone placed on ice. The samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. E1 trimerization was determined by autoradiography and phosphorimaging analysis. The numbers of counts in the trimer band are shown as percentages of the total amount of E1 present.