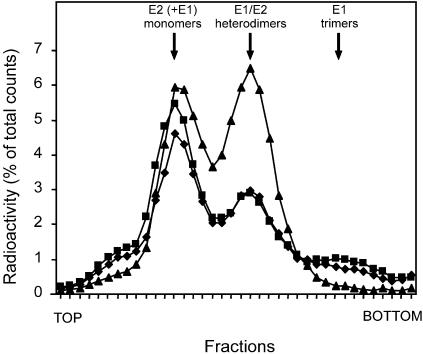
FIG. 5.
Effects of acidification and neutralization on E1/E2 heterodimer dissociation. Dissociation of the E1/E2 heterodimer was determined under the same experimental conditions as in the fusion and binding experiments. Radioactively labeled SFV (105 cpm) in HNE buffer (pH 7.4) in a glass tube was continuously stirred at 37°C. Squares represent SFV acidified for 5 min by addition of a small titrated volume 0.1 M MES and 0.2 M acetic acid. Subsequently, the virus was solubilized by addition of NP-40 (to a 1% final volume), and the mixture was neutralized (to a final concentration of 1%) by addition of a small, pretitrated volume of 0.1 M NaOH. Diamonds represent SFV acidified as described above. After 5 min, the pH of the sample was raised to pH 8 by the addition of a small, pretitrated volume of 0.1 M NaOH. Subsequently, NP-40 (1%) was added for solubilization of the virus. Triangles represent SFV in HNE buffer (pH 7.4) solubilized by the addition of NP-40 (1%). The samples were layered on top of a 5 to 20% (wt/wt) continuous sucrose gradient in HNE buffer (pH 7.4) and 0.1% NP-40 in a Beckman SW50 centrifuge tube. After centrifugation in a Beckman SW50 rotor at 192,000 × g for 16 h at 4°C, the gradients were fractionated in 38 fractions from the top. The radioactivity in each fraction was determined by liquid scintillation analysis.