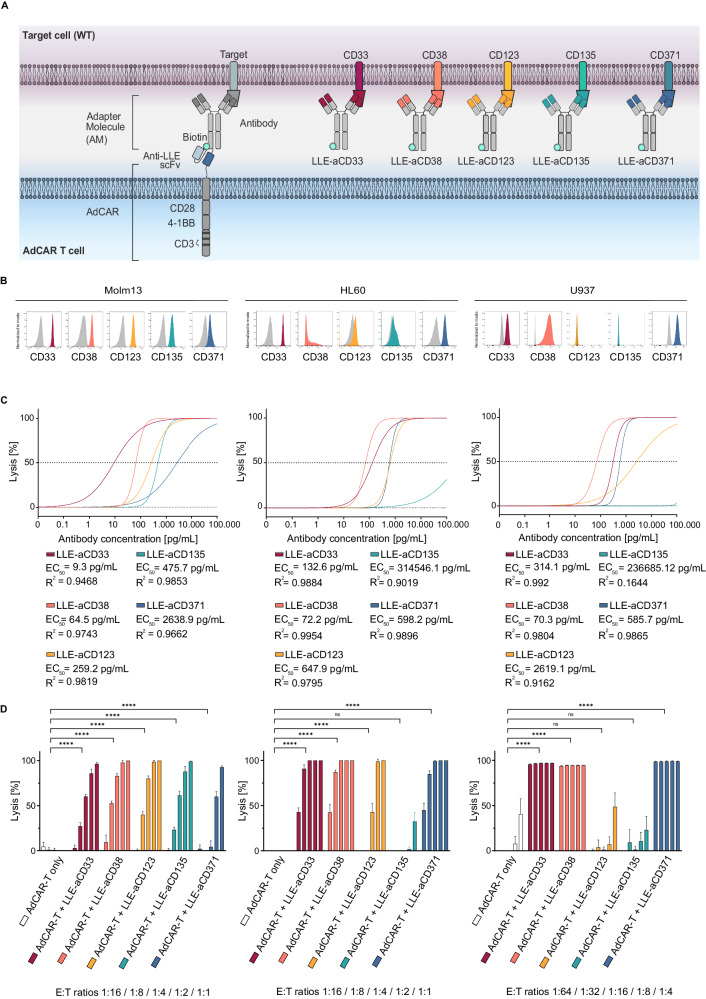
Fig. 2. Design and in vitro evaluation of novel AML-targeted AMs.
A Schematic illustration of the AdCAR-T system. AdCAR-T cells are directed to AML-associated target antigens via LLE-conjugated mAbs (LLEa-CD33, LLEa-CD38, LLEa-CD123, LLEa-CD135, and LLEa-CD371), referred to as AMs. B Histograms of target antigen expression (CD33, CD38, CD123, CD135 and CD371) on AML cell lines (Molm13, HL60 and U937) stained with the indicated AM and secondary anti-LLE mAb, analyzed by flow cytometry. C Cytotoxicity of AdCAR-T against the indicated luciferase-expressing AML cell lines as determined by LCA after 48 h at an E:T of 1:1 (Molm13, HL60) or 1:4 (U937), mediated by increasing concentrations, logarithmic titration steps from 0.1 pg/mL to 100 ng/mL, of the indicated AMs. EC50 values are provided for each AM. D Cytotoxicity of AdCAR-T cells against the indicated AML cell lines as determined by LCA after 48 h at fixed AM concentrations (10 ng/mL) and indicated E:T ratios. AdCAR-T cells in the absence of AM served as a negative control. Data shown in C and D represent the mean ± SD of (n = 6). Data shown in C were transformed by taking the logarithm of the x values and then fitted by nonlinear regression. Statistical analysis was performed using two-way ANOVA and Tukey’s multiple comparison test. ns, not significant. *p ≤ 0.0332 **p ≤ 0.021. ***p ≤ 0.002. ****p ≤ 0.0001. The full table of the statistical analysis is provided in Additional file 1.