Abstract
Herpes simplex virus (HSV) enters some laboratory cell lines via a pH-dependent, endocytic mechanism. We investigated whether this entry pathway is used in human cell types relevant to pathogenesis. Three different classes of lysosomotropic agents, which raise endosomal pH, blocked HSV entry into primary and transformed human keratinocytes, but not into human neurons or neuroblastoma lines. In keratinocytes, incoming HSV particles colocalized with markers of endocytic uptake. Treatment with the isoflavone genistein, an inhibitor of protein tyrosine kinases, reduced the delivery of incoming viral particles to the nuclear periphery and virus-induced gene expression in keratinocytes but not neurons. Moreover, in keratinocyte monolayer islets, HSV infected both the inner and outer cells in a genistein-sensitive manner, suggesting viral endocytosis from both basolateral and apical plasma membrane surfaces. Together, the results indicate that HSV enters human epidermal keratinocytes, but not neurons, by a low-pH, endocytic pathway that is dependent on host tyrosine phosphorylation. Thus, HSV utilizes fundamentally different cellular entry pathways to infect important target cell populations.
Herpes simplex virus (HSV) infects many cell types. The major target cells during primary and recurrent HSV infection, however, are cells of epithelial and neuronal origin (37). During initial exposure, HSV uses mucosal epithelial cells, including epidermal keratinocytes, as the primary portal of entry and spreads through the epithelium. Virions then infect the axon terminals of sensory neurons that innervate the superficial dermis. HSV travels by retrograde axonal transport to the neuronal cell body. At that point, the virus can abandon the replicative process and establish a latent infection. Following episodic reactivation, newly replicated HSV is transported back to the axonal termini. From there it spreads to infect epithelial cells, often leading to a recurrent herpetic lesion.
This constitutes the classically defined route of infection in the normal host. However, in neonates and immunocompromised individuals, HSV can escape immune containment and disseminate to infect numerous additional cell types and organ systems, including the brain (52). HSV also exhibits a very wide cellular host range in vitro and in animal models. Viral entry into this broad array of host cell types may be facilitated by multiple cellular pathways.
The majority of animal virus families take advantage of endocytosis to accomplish cell entry (34). For many years, it was thought that HSV enters cells exclusively by fusing with the cell membrane with no requirement for endocytosis. Recently, we demonstrated that HSV entry into cultured cells can proceed via endocytic as well as nonendocytic mechanisms. Active endocytosis is necessary for HSV entry into Chinese hamster ovary (CHO) cells that express the gD-binding entry receptors HVEM, nectin-1, or nectin-2 and HeLa cells (32). In contrast, entry into other cultured cell types, such as Vero, occurs by direct penetration of the plasma membrane and has no apparent requirement for endocytosis (14, 32, 33, 54).
Both the endocytic and nonendocytic entry pathways share a number of features. Study of the kinetics of initial uptake, trafficking, penetration, and virion capsid delivery to the nucleus indicated that entry by an endocytic mechanism is rapid and efficient and leads to productive infection (33), as is the case for direct penetration at the cell surface. The completion of the entry process via either pathway requires participation of envelope glycoproteins gB and gD and the gH-gL heterodimer (33, 42).
Binding of virion gD to any one of its cognate receptors is a required component of the HSV entry process (7, 8, 41). In the nonendocytic pathway, HSV engages gD receptors at the cell surface and the capsid penetrates directly into the cytosol. In the endocytic entry pathway, capsid penetration is spatially distinct from cell surface binding. The enveloped virion is first taken up from the cell surface in a process termed internalization. This step is essential for successful endocytic entry but does not occur in the case of direct penetration at the plasma membrane. Internalization of HSV is rapid but is not mediated by any of the known gD receptors (33). Endocytosed HSV traverses a lysosome-terminal endosomal pathway. Trafficking of the virus to the site of intracellular penetration is also independent of gD receptors. However, interaction with a gD receptor, either at the plasma membrane or at an internal membrane, is required for escape of the capsid from the endosome into the cytosol. In the absence of receptor interaction, virions are trapped within endocytic compartments and ultimately undergo lysosomal degradation (33).
Common properties of viruses that utilize pH-dependent entry pathways include (i) entry by an endocytic mechanism, (ii) requirement of endosomal low pH for entry; (iii) inactivation of entry function by low-pH pretreatment of isolated particles, and (iv) activation of membrane fusion function by acid pH (13). HSV fulfills the first three of these four criteria, at least in certain cell types (32, 33). HSV entry into cells that support an endocytic entry pathway is susceptible to inhibition by lysosomotropic agents, which elevate the normally acidic pH of endosomes (32). However, HSV penetration at the surface (of Vero cells, for example) is not inhibited by such treatment (23, 32, 54) and is considered pH independent. Consistent with a role for pH in membrane fusion, treatment of purified HSV particles with a mildly acidic pH of 4.5 to 5.5 irreversibly inactivates entry activity (32). Inactivation is temperature dependent and does not require receptor interaction. However, transfection of cells with gB, gD, and gH-gL induces cell fusion that proceeds at neutral pH (30, 48). This seeming incongruity may be explained by a model in which HSV has the capacity to initiate both pH-dependent and pH-independent membrane fusion (32). It is also important to note that while the processes of cell fusion and virus-cell fusion during entry have similarities (4, 30, 36), they are not identical (6, 30, 53). Interestingly, HSV entry into a BHK cell variant mediated by nectin-1 is not inhibited by lysosomotropic agents, whereas entry mediated by either nectin-1 fused to epidermal growth factor receptor sequences or glycosylphosphatidylinositol-anchored nectin-1 is inhibited (15).
Here we determined that HSV utilizes distinct cellular pathways to enter human keratinocytes and neurons. Specifically, we found that: (i) HSV entry into primary and transformed epidermal keratinocytes, but not neuronal cells, is inhibited by agents that elevate endosomal pH; (ii) incoming virions colocalize with markers of fluid-phase endocytosis in keratinocytes; and (iii) cellular tyrosine kinase activity is selectively required for efficient entry by the low-pH, endocytic pathway.
(This work was presented in part at the 29th International Herpesvirus Workshop in Reno, Nev., 25 to 30 July 2004, abstr. 2.03).
MATERIALS AND METHODS
Cells and viruses.
HaCaT keratinocytes (3) (provided by Harvey Friedman, University of Pennsylvania) were propagated in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, Calif.) supplemented with 10% fetal bovine serum (Invitrogen). Normal adult primary human epidermal keratinocytes (NHEK; Cambrex, Walkersville, Md.) were propagated in complete keratinocyte growth medium (Cambrex). SCC13 cells (11) (squamous cell carcinoma cells provided by Colette Cywes, Harvard University) were propagated in serum-free keratinocyte growth medium (Invitrogen) supplemented with 0.1 ng/ml epidermal growth factor, 50 μg/ml bovine pituitary extract, and 0.3 mM CaCl2. The neuroblastoma cell lines SH-SY5Y, IMR-32, and SK-N-SH (American Type Culture Collection, Rockville, Md.) were propagated in Eagle's minimal essential medium supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and Earle's salts (Invitrogen).
Human central nervous system progenitor cells were isolated from an 8-week-gestation fetal brain according to National Institutes of Health guidelines. The isolation and culture conditions of the progenitor cells and subsequent selective differentiation into neurons were previously reported (27). Progenitors were differentiated into a neuronal phenotype (MAP-2+, βIII-tubulin+, and negative for nestin) by culturing in 10 ng/ml brain-derived neurotrophic factor and 10 ng/ml platelet-derived growth factor A/B (Sigma, St. Louis, Mo.).
The HSV-1 strain KOS derivative 7134 contains the Escherichia coli lacZ gene in place of the immediate-early ICP0 gene (5) (provided by Priscilla Schaffer, Harvard University). HSV-1 KOS K26GFP contains green fluorescent protein (GFP) fused to the N terminus of the VP26 capsid protein (12) (provided by Prashant Desai, Johns Hopkins University). Viruses were propagated and titers were determined on Vero cells.
Uptake of infectious HSV from the cell surface.
The assay was performed essentially as described previously (33). HSV-1 KOS K26GFP was bound to cells and incubated at 37°C for various times up to 1 h. Extracellular virus was acid inactivated. At 8 h postinfection (hpi), cells were fixed in 3% paraformaldehyde. Nuclei were stained with 25 ng/ml 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI; Roche Diagnostics, Indianapolis, Ind.) to determine the total cell number, and GFP-positive nuclei were counted.
β-Galactosidase reporter assay.
Confluent cell monolayers grown in 96-well dishes were infected with HSV-1 7134 at a multiplicity of infection (MOI) of 1. Cells were treated with a range of concentrations of inhibitors or were mock treated at 37°C for 1 h prior to addition of virus. Infected cells were incubated at 37°C in the presence or absence of inhibitor for 7 h. Cells were lysed in 0.5% Nonidet P-40, chlorophenol red-β-d-galactopyranoside (Roche Diagnostics) was added, and then β-galactosidase activity was determined by absorbance at 560 nm using a microtiter plate reader (Dynatech, Chantilly, Va.). Mean results were calculated for four replicate samples. The β-galactosidase activity from infected but mock-treated cells was defined as 100%.
Inhibition of HSV plaque formation.
HaCaT or SK-N-SH monolayers were treated with culture medium containing agent or mock treated for 1 h at 37°C. Ten-fold dilutions of HSV-1 KOS were added to cells for 6 h at 37°C. Medium was removed, and cultures were washed, twice with phosphate-buffered saline (PBS) and once with medium, and then normal culture medium was added. Cultures were incubated for an additional 16 h. Cells were fixed with ice-cold methanol-acetone solution (2:1 ratio) for 20 min at −20°C and air dried. Virus titers were determined by an immunoperoxidase assay (45) using anti-HSV polyclonal antibody HR50 (Fitzgerald Industries, Concord, Mass.).
Adsorption of radiolabeled HSV to the cell surface.
As previously described (33), HSV labeled with [35S]cysteine and [35S]methionine was prepared by harvesting infected cell supernatant and then pelleting the extracellular virus by centrifugation at 27,000 × g for 45 min at 4°C. HaCaT cells were treated with various concentrations of inhibitors or mock treated for 1 h at 37°C. Radiolabeled HSV (specific activity of 50 cpm/PFU) was added to cells in Dulbecco's modified Eagle's medium buffered with 20 mM HEPES for 1 h at 4°C in the presence or absence of inhibitors. Cells were washed three times with PBS, and then 0.5% Nonidet P-40 lysates were prepared. Radioactivity was quantified in a liquid scintillation counter. Cell-associated cpm represented virus bound to the cell surface.
Fluorescence microscopy of HSV transport to the nucleus.
The assay was performed essentially as described previously (32). HSV-1 KOS K26GFP was added to cells for various times in the presence or absence of inhibitors. Cells were fixed with 3% paraformaldehyde and permeabilized with 0.2% Triton X-100. Nuclei were counterstained with DAPI. Cells were viewed with a Zeiss Axioplan 2 imaging microscope. Digital images were captured with a Zeiss AxioCam using Openlab 3.1 software (Improvision, Lexington, Mass.) and processed with Adobe Photoshop 6.0.
Colocalization of incoming HSV with dextran-Texas red.
HaCaT or SK-N-SH cells were grown on glass coverslips overnight. Cells were chilled for 15 min, and then HSV-1 KOS K26GFP was added at an MOI of 20 for 2 h at 4°C. Cells were washed three times with PBS, then warmed medium containing 1 mg/ml 70,000-kDa dextran-Texas red (Molecular Probes, Eugene, Oreg.) was added, and the cells were rapidly warmed to 37°C. HSV infection did not alter dextran uptake (data not shown). At 12 or 45 min postinfection (p.i.), culture plates were transferred to ice and then washed three times with ice-cold PBS. Cells were fixed with 3% paraformaldehyde in PBS and viewed with a Leica TCS NT inverted confocal microscope. Images were processed with Adobe Photoshop 6.0.
Infection of keratinocyte islet cultures.
A single-cell suspension of HaCaT cells was seeded onto glass coverslips. Discrete islet monolayers of ∼20 to 25 cells each formed in culture by 3 to 4 days. Cultures were treated with 50 μM genistein or mock treated for 1 h and were infected with HSV-1 KOS K26GFP at an MOI of 0.5 or 5 for 7 h in the continued presence or absence of genistein. Cultures were fixed with 3% paraformaldehyde, permeabilized, and then counterstained with DAPI.
RESULTS
Lysosomotropic agents inhibit HSV infection of human keratinocytes but not neuronal cells.
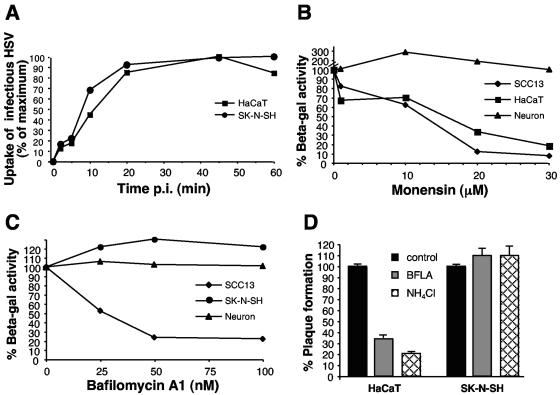
We previously characterized the low-pH, endocytic pathway used by HSV for entry into CHO cells expressing gD receptors and HeLa cells. To determine whether more pathophysiologically relevant cell types support pH-dependent entry, the effects of lysosomotropic agents on entry of HSV into human keratinocyte and neuronal cell lines were examined. Prior to assessing the time-dependent effects of these inhibitors, the rates of virus entry into each cell type were determined. To this end, the kinetics of HSV uptake from the surface of HaCaT keratinocytes or SK-N-SH neuroblastoma cells was determined by acquisition of resistance to acid inactivation as described previously (33). Infectious HSV was rapidly taken up from the surface of both cell types, with a t1/2 of ∼8 to 11 min (Fig. 1A). Uptake of bound HSV reached maximum by 45 min in both the keratinocyte and neuroblastoma lines, indicating similar kinetics of surface uptake of infectious HSV. The plaquing efficiency was also similar on each cell type (data not shown).
FIG. 1.
Effect of lysosomotropic agents on HSV infection of neurons and keratinocytes. (A) Uptake of infectious HSV from the cell surface. HSV-1 KOS K26GFP was bound to HaCaT keratinocytes or SK-N-SH neuroblastoma cells for 1 h at 4°C (MOI of 0.5). Cells were washed with PBS and incubated at 37°C, and extracellular virus was inactivated by acid treatment at the indicated times. At 8 hpi, cells were fixed and random fields of ∼1,000 cells in total were evaluated per sample. Cell number was determined by nuclear staining with DAPI, and infected, GFP-positive cells were counted. Maximum infectivity was set to 100%. (B, C) Effect of lysosomotropic agents on HSV-induced gene expression. Cells were pretreated with the indicated concentrations of agent for 1 h. Cells were infected with the lacZ+ HSV-1 strain KOS 7134 at an MOI of 1 for 7 h in the continued presence of agent. (D) Effect of lysosomotropic agents on HSV plaque formation. Cells were pretreated with 100 nM bafilomycin or 50 mM ammonium chloride for 1 h. HSV-1 KOS was added for 6 h at 37°C in the presence of the agent. The monolayers were washed and then incubated in normal medium for an additional 16 h. Plaques were visualized by immunoperoxidase staining with anti-HSV polyclonal sera. Each point represents the mean of quadruplicate wells.
Next, HSV-1 KOS 7134, which contains the β-galactosidase gene under the viral ICP0 promoter (5), was added to various cells for 7 h in the presence or absence of the carboxylic ionophore monensin or the vacuolar H+-ATPase inhibitor bafilomycin A1. Such lysosomotropic agents elevate the normally low pH of intracellular compartments by distinct mechanisms (26). As in prior studies using other cell lines (32), these agents did not affect cell viability under the conditions tested (data not shown). When HaCaT or SCC13 keratinocyte lines were treated with monensin or bafilomycin A1, there was a concentration-dependent inhibition of HSV-induced β-galactosidase activity (Fig. 1B and C). In contrast, at all concentrations tested there were no inhibitory effects of these agents on β-galactosidase expression in SK-N-SH cells or human central nervous system progenitor-derived neurons (Fig. 1B and C).
The analysis of the effects of pH-altering agents was extended to include normal primary human keratinocytes (NHEK) and two additional human neuroblastoma cell lines: IMR32, and SH-SY5Y (Table 1). HSV entry into NHEK cells was inhibited by the weak base, ammonium chloride, and by monensin. In contrast, entry into each of the neuroblastoma cell lines was not inhibited by these treatments (Table 1). In fact, in several instances, a relative enhancement of entry into treated neuroblastoma cells was observed.
TABLE 1.
Effect of lysosomotropic agents on HSV entry into human cells
| Cell | Type | % β-Galactosidase activity
|
|
|---|---|---|---|
| + NH4Cl (50 mM) | + Monensin (30 μM) | ||
| IMR32 | Neuroblastoma | 134.1 ± 10.1 | 115.7 ± 10.8 |
| SH-SY5Y | Neuroblastoma | 453.6 ± 93.7 | 154.4 ± 93.8 |
| SK-N-SH | Neuroblastoma | 93.0 ± 10.1 | 213.0 ± 10.2 |
| NHEK | Primary keratinocyte | 0.8 ± 4.2 | 49.0 ± 7.8 |
To further establish the low-pH requirement for entry of HSV into keratinocytes, the effect of lysosomotropic agents on HSV-1 KOS plaque formation was measured at 22 hpi. When bafilomycin A1 or ammonium chloride was present in the culture for the first 6 h of infection, plaque formation on HaCaT keratinocytes was notably inhibited (Fig. 1D, left). Similar treatment had no effect on plaques formed on SK-N-SH cells (Fig. 1D, right). Thus, both entry and subsequent replication of HSV in primary and transformed keratinocytes are pH dependent.
Low intracellular pH is necessary for delivery of HSV to the nuclear periphery of keratinocytes.
Having shown that lysosomotropic agents inhibit HSV gene expression in keratinocytes at 7 hpi, we sought to demonstrate that an early step in the entry process was affected. First, we determined the effect of lysosomotropic agents on HSV adsorption to keratinocytes. None of the lysosomotropic agents bafilomycin A1, monensin, or ammonium chloride inhibited attachment of radiolabeled HSV to the surface of HaCaT cells relative to untreated cells (Fig. 2). As a positive control, heparin effectively blocked virus binding (Fig. 2).
FIG. 2.
Binding of HSV to the cell surface in the presence of various agents. HaCaT cells were treated for 1 h at 37°C with 30 μM monensin, 50 mM ammonium chloride, 100 nM bafilomycin A1 (BFLA), 200 μM genistein or 1 μg/ml heparin. 35S-labeled HSV-1 KOS was added (MOI of 3) in the continued presence of agents for 1 h at 4°C. Cells were washed with PBS, detergent lysates were prepared, and samples were analyzed with a liquid scintillation counter. Cell-associated cpm represents virus that was bound to the cell surface. The mean of three replicate samples ± standard error is shown.
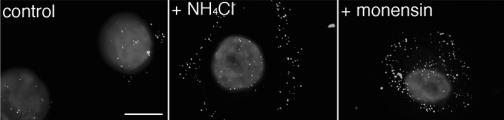
To initiate infection, incoming HSV must travel to the nucleus, the site of replication. Thus, if lysosomotropic agents prevent virions from penetrating from an endosome, these agents should alter the subcellular distribution of HSV early in infection (32). Therefore, localization of incoming GFP-tagged HSV was monitored by fluorescence microscopy. In the presence of ammonium chloride or monensin, virions were distributed primarily in the cytoplasm at 3 hpi, while in untreated controls, particles accumulated at or around the nucleus, as expected (Fig. 3). Fewer fluorescent particles were seen in untreated control cells, likely because virion capsids dissociate following release of their viral DNA into the nucleus and are no longer detectable. In this assay, lower concentrations of agent inhibited virus entry at 3 hpi (Fig. 3) than were required for inhibition of gene expression at 7 hpi (Fig. 1B and Table 1). This has been seen previously with bafilomycin and ammonium chloride (32) and also holds true for wortmannin (33) and genistein (see Fig. 5). Together, the results indicate that intracellular low pH is required for a step in HSV infection that occurs after particles bind to the cell surface but prior to their arrival at the nucleus.
FIG. 3.
Intracellular low pH is important for HSV entry into keratinocytes. Primary human keratinocytes were treated with 10 mM ammonium chloride, 5 μM monensin, or no agent for 1 h at 37°C. HSV-1 KOS K26GFP (MOI of 20) was then bound to the cells for 2 h at 4°C. Cells were washed with PBS, and then warmed medium containing the indicated inhibitors was added. Infection proceeded for 3 h in the presence of cycloheximide. Cells were washed with PBS and fixed in 3% paraformaldehyde. Nuclei were counterstained with DAPI. Punctate fluorescence indicates HSV particles. Cells were viewed with a ×63 oil immersion objective. The images are representative of the cell population. Bar, 10 μm.
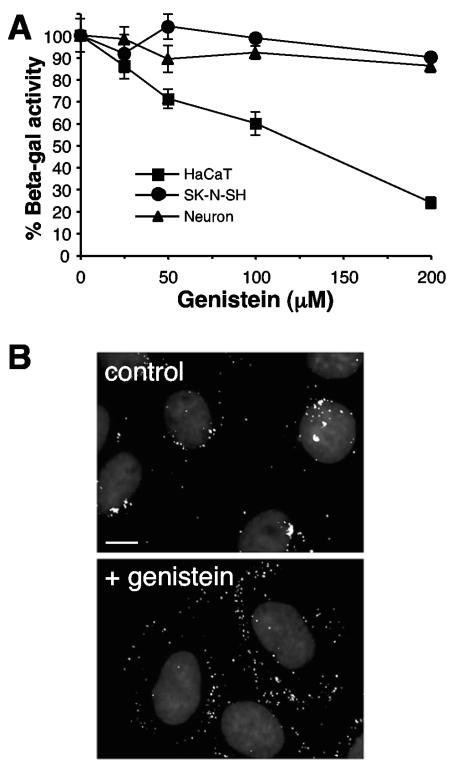
FIG. 5.
Role of host tyrosine phosphorylation in HSV entry and virus-induced gene expression. (A) HaCaT, fetal brain progenitor-derived neurons, or SK-N-SH cells were pretreated with the indicated concentrations of genistein for 1 h. Cells were infected with lacZ+ KOS 7134 at an MOI of 1 for 7 h in the continued presence of genistein. Entry was measured as the percentage of β-galactosidase activity relative to that obtained in the absence of genistein. Standard deviations are indicated. (B) Cells were treated for 1 h with 20 μM genistein (lower panel) or untreated (upper panel), and then HSV-1 KOS K26GFP (MOI of 20) was bound at 4°C for 2 h. Following a shift to 37°C for 2 h in the presence of genistein, cells were processed as described in the legend to Fig. 3. The images are representative of the cell population. Bar, 10 μm.
Partial colocalization of incoming virions with markers of fluid-phase endocytosis.
The pH sensitivity of HSV entry into and infection of keratinocytes suggests that infectious virus is exposed to the acidic environment of an intracellular compartment. Thus, it is likely that the virus is first taken up into the cell by an endocytic mechanism. To document this, we sought to colocalize entering particles with endocytic compartments. Cells efficiently internalize fluid and solutes from the extracellular space by fluid-phase uptake, which includes receptor-mediated endocytic events (10). Uptake of labeled dextran from the extracellular medium is an indicator of bulk flow endocytosis. Dextran is useful in this regard because it does not stimulate endocytosis and has low nonspecific binding to the cell surface (1).
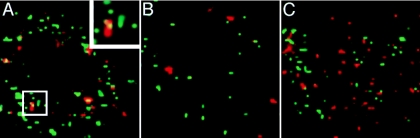
Localization of GFP-tagged HSV particles relative to subcellular compartments containing dextran-Texas red was determined during infection of keratinocytes and neuroblastoma cells using laser-scanning confocal microscopy (Fig. 4). In HaCaT cells, some incoming HSV particles colocalized with dextran at 12 min p.i. in punctate intracellular structures (Fig. 4A). Much of the GFP signal, however, did not colocalize with dextran-positive compartments. There are a few possible reasons for this. First, the efficiency of HSV uptake by endocytosis is ∼50 to 60%, which means that a nearly equivalent fraction of virus that is bound to the cell surface is never internalized (33). Second, by 12 min p.i., some infectious virus has not yet entered into dextran-staining vesicles, whereas, some fraction of virus has already penetrated into the cytosol by this point. Third, by 45 min p.i., the relative amount of GFP-labeled virus present in dextran-positive compartments had already diminished greatly (Fig. 4B). This indicates that virions had exited from these vesicles to continue the entry process. In contrast, little or no colocalization of HSV with dextran-containing compartments was observed in SK-N-SH cells at 12 min (Fig. 4C) or 45 min p.i. (data not shown).
FIG. 4.
Colocalization of incoming HSV with dextran, a marker of fluid-phase endocytosis. HaCaT (A, B) or SK-N-SH (C) cells were chilled for 15 min, and then HSV-1 KOS K26GFP was added for 2 h at 4°C. Cells were washed, and then warmed medium containing 1 mg/ml 70,000-kDa dextran-Texas red was added, and cells were rapidly warmed to 37°C. At 12 min (A, C) or 45 min (B) p.i., cells were fixed with 3% paraformaldehyde. A confocal slice of a single cell is shown for each. The inset in panel A is a magnification of the boxed area. Bar, 5 μm.
Tyrosine kinase activity is necessary for endocytic entry of HSV in keratinocytes.
Endocytosis is a tightly regulated process involving host kinase activities. It was previously shown that the lipid kinase, phosphatidylinositol 3-kinase (PI 3-kinase) was needed for entry into cells that support endocytic entry such as CHO-nectin-1 and HeLa cells (33). To further probe the molecular requirements for endocytic entry of HSV, we tested the effects of kinase inhibitors on entry into keratinocytes and neurons. Both tyrosine kinase and PI 3-kinase activities are needed for phosphatidylinositol turnover (49). The isoflavone genistein (4′,5,7-trihydroxyisoflavone) is a well-characterized inhibitor of tyrosine kinases (43, 51). HSV entry into HaCaT cells, but not SK-N-SH cells or progenitor-derived neurons, was inhibited by genistein, as measured by virus-induced β-galactosidase activity (Fig. 5A). In addition to inhibiting tyrosine kinase, however, genistein may also reduce cell proliferation, modify the cell cycle, and induce cell differentiation (43, 51). Thus, it was important to demonstrate more specifically at which stage of HSV infection genistein was acting. Treatment of HaCaT cells with genistein blocked the arrival of incoming GFP-tagged HSV at the nucleus as compared to untreated infected cells (Fig. 5B). Similar results were obtained with wortmannin, an inhibitor of PI 3-kinase (33; data not shown). Moreover, genistein had little inhibitory effect on binding of radiolabeled HSV to keratinocytes (Fig. 2). These data suggest a role for host tyrosine phosphorylation at an early stage of entry by endocytosis. This further distinguishes HSV entry into epithelial cells from entry into neuronal cells.
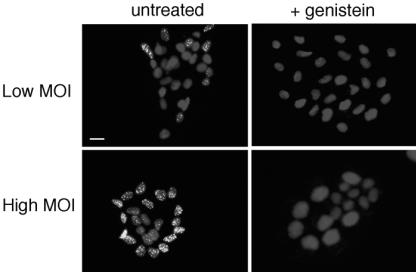
Genistein inhibits HSV infection of keratinocyte islets.
Keratinocytes are highly polarized in vivo and in vitro. Many cell and tissue culture systems have been used to study the polarity of HSV infection of the epithelium (17, 35, 38, 39, 44, 50). We used a two-dimensional keratinocyte islet model to probe further the role of tyrosine phosphorylation in endocytic entry of HSV. In this system, cells are plated at low density and allowed to form clustered monolayers of cells or islets over several days. The outer rim of cells in the islet has basolateral surfaces exposed, whereas the inner cells have only the apical surfaces exposed and available for entry.
We investigated the requirement for tyrosine phosphorylation during infection of HaCaT cell islets. At low MOI, HSV K26GFP preferentially infected the outer cells of the islet, as indicated by detection of newly synthesized VP26-GFP in the nucleus at 7 hpi (Fig. 6A). The preference of HSV for infecting exterior cells of Madin-Darby canine kidney cell islets has been noted previously (25, 38). At high MOI, however, HSV entered both the outer and inner cells, albeit with less VP26 detected in the interior cells (Fig. 6B). Additionally, HSV entered all cells of a fully confluent HaCaT cell monolayer when infected at a high MOI (data not shown). Pretreatment of HaCaT islets with genistein inhibited infection at both low and high multiplicities (Fig. 6C and D). Taken together, the data suggest that HSV has the capacity to infect HaCaT cells via both basolateral and apical plasma membrane domains and that entry occurs via genistein-sensitive endocytosis.
FIG. 6.
Effect of genistein on infection of keratinocyte islets. HaCaT cells were seeded at low density on glass coverslips. Islets formed by 3 to 4 days of culture. Cultures were treated with 50 μM genistein or mock treated for 1 h and were infected with HSV-1 KOS K26GFP at an MOI of 0.5 (low) or 5 (high) for 7 h in the presence or absence of genistein. Similar results were obtained with wortmannin (data not shown). Cultures were fixed with 3% paraformaldehyde, permeabilized, and then counterstained with DAPI. Newly synthesized VP26-GFP in the nucleus indicates successful entry and viral protein synthesis. Cells were viewed with a ×20 objective. Bar, 20 μm.
DISCUSSION
Incoming HSV can utilize endocytic and nonendocytic mechanisms to deliver its genome to host cells. Here, we provide evidence that both pathways are relevant to viral pathogenesis by demonstrating their importance for entry into specific human cells. HSV uses a pH-dependent, endocytic pathway to enter primary human keratinocytes, initial targets of HSV infection in vivo. The virus may require endocytosis to bypass the dense layer of cortical actin just under the plasma membrane of epithelial cells. This route of entry is sensitive to genistein and wortmannin and is used when HSV infects either the apical or basolateral plasma membrane surfaces. Thus, the endosomal machinery of epithelial cells may provide a previously unrecognized opportunity for intervention at the portal of HSV entry into the human host. In contrast, HSV entry into human neuronal cells proceeded via a pH-independent pathway that is not affected by the kinase inhibitors tested.
Keratinocytes are epithelial cells that comprise the skin and mucosa and provide a barrier between host and environment. In vivo, epidermal keratinocytes represent the site of initial exposure as well as the main target of reactivating virus. In culture, keratinocytes are highly susceptible to HSV infection (18, 20, 22, 35). Studies of organotypic raft cultures of keratinocytes have been used to mimic infection of stratified epithelia (19, 28, 38, 44, 50). Also, keratinocytes have been used as a model for HSV spread in epithelial cells (18, 22).
Previous studies have reported on HSV infection of epithelial cells by either the basolateral surface (25, 38) or by both the apical and basolateral surfaces (16, 17, 25, 39, 46, 47, 55). HSV can apparently enter HaCaT keratinocytes from either the apical or the basolateral surfaces by endocytosis, although the basolateral domain seems to be preferred at low MOI (Fig. 5 and 6). HSV infection of Madin-Darby canine kidney (MDCK) cells is facilitated by disruption of cell junctions, either by mechanical wounding (17, 38) or by calcium depletion (17, 25, 46, 55), suggesting that HSV also favors the basolateral surfaces of MDCK cells.
Infection of neuronal cells permits HSV latency and reactivation. Human neurons proved to be distinct from keratinocytes in that they support an HSV entry pathway that is not inhibited by lysosomotropic agents that elevate endosomal pH (Fig. 1 and Table 1). This is consistent with ultrastructural analysis of HSV infection of neurons that revealed fusion of virus particles with the cell surface (24). In neuroblastoma lines and other cells that support pH-independent entry of HSV, lysosomotropic agents seem to enhance entry, particularly when relatively late events such as gene expression are measured (Fig. 1 and Table 1) (32, 57). In addition to elevating endosomal pH, which prevents viral penetration in some cell types, lysosomotropic agents also incapacitate the acid-dependent degradative enzymes of the lysosome. Thus, in treated neuronal cells, a portion of the inoculum that would normally be degraded may have an extended opportunity to penetrate (Fig. 1 and Table 1).
Several features of the endocytic pathway used by HSV to enter keratinocytes, HeLa cells, and CHO cells suggest that it involves macropinocytosis. Macropinocytosis is a clathrin-independent process characterized by large, heterogeneous vesicular structures called macropinosomes caused by the closure of lamellipodia (10, 34). HSV is detected in large (0.3 to 1 μm in diameter), smooth-walled vesicles at early times during endocytic entry (32, 33). The role of clathrin in the uptake of HSV remains to be determined directly. Macropinocytosis is thought to be somewhat nonspecific, and cargo molecules of macropinosomes are not well defined. Macropinocytosis is used by cells to internalize large amounts of fluid and membrane; therefore, the fluid-phase marker dextran can been used to label macropinocytic vesicles (21, 56). In this regard, HSV is partially localized to dextran-positive compartments at 12 min p.i. (Fig. 4). Moreover, formation of macropinosomes is blocked by inhibitors of PI 3-kinase (40). Endocytic trafficking of HSV is impaired by two inhibitors of PI 3-kinase, wortmannin and LY294002 (33). Protein tyrosine kinase activity is necessary for PI 3-kinase function. The tyrosine kinase inhibitor genistein blocks endocytic entry into keratinocytes (Fig. 5 and 6). The kinase inhibitor studies suggest that the phosphatidylinositol turnover pathway is involved in endocytic trafficking of HSV. In all, the data implicate macropinosomes in HSV entry, but elucidation of their role awaits more analysis.
HSV-1 and HSV-2 are not the only human herpesviruses to utilize alternative entry pathways in a cell-type-dependent manner. The gammaherpesvirus Epstein-Barr virus enters epithelial cells via fusion at the plasma membrane, yet it requires endocytosis for entry into B cells (29, 31). Human cytomegalovirus, a betaherpesvirus, fuses at the plasma membrane of fibroblasts (2, 9) but enters epithelial cells by endocytosis (2). Herpesviruses may have evolved to exploit distinct entry mechanisms to invade their physiologically relevant target cells.
Acknowledgments
We thank Colette Cywes, Prashant Desai, and Harvey Friedman for reagents used in this study. We are grateful to Jeffrey Cohen and Qingxue Li for critical reading of the manuscript.
REFERENCES
- 1.Berlin, R. D., and J. M. Oliver. 1980. Surface functions during mitosis. II. Quantitation of pinocytosis and kinetic characterization of the mitotic cycle with a new fluorescence technique. J. Cell Biol. 85:660-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodaghi, B., M. E. Slobbe-van Drunen, A. Topilko, E. Perret, R. C. Vossen, M. C. van Dam-Mieras, D. Zipeto, J. L. Virelizier, P. LeHoang, C. A. Bruggeman, and S. Michelson. 1999. Entry of human cytomegalovirus into retinal pigment epithelial and endothelial cells by endocytosis. Investig. Ophthalmol. Vis. Sci. 40:2598-2607. [PubMed] [Google Scholar]
- 3.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82:1419-1422. [DOI] [PubMed] [Google Scholar]
- 5.Cai, W., and P. A. Schaffer. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J. Virol. 65:4078-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairns, T. M., R. S. Milne, M. Ponce-de-Leon, D. K. Tobin, G. H. Cohen, and R. J. Eisenberg. 2003. Structure-function analysis of herpes simplex virus type 1 gD and gH-gL: clues from gDgH chimeras. J. Virol. 77:6731-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. [DOI] [PubMed] [Google Scholar]
- 8.Carfí, A., S. H. Willis, J. C. Whitbeck, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol. Cell 8:169-179. [DOI] [PubMed] [Google Scholar]
- 9.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191:387-395. [DOI] [PubMed] [Google Scholar]
- 10.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 11.Cywes, C., and M. R. Wessels. 2001. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature 414:648-652. [DOI] [PubMed] [Google Scholar]
- 12.Desai, P., and S. Person. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 72:7563-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earp, L. J., S. E. Delos, H. E. Park, and J. M. White. 2004. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 285:25-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller, A. O., and P. G. Spear. 1987. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc. Natl. Acad. Sci. USA 84:5454-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianni, T., G. Campadelli-Fiume, and L. Menotti. 2004. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J. Virol. 78:12268-12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths, A., S. Renfrey, and T. Minson. 1998. Glycoprotein C-deficient mutants of two strains of herpes simplex virus type 1 exhibit unaltered adsorption characteristics on polarized or non-polarized cells. J. Gen. Virol. 79:807-812. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi, K. 1995. Role of tight junctions of polarized epithelial MDCK cells in the replication of herpes simplex virus type 1. J. Med. Virol. 47:323-329. [DOI] [PubMed] [Google Scholar]
- 18.Huber, M. T., T. W. Wisner, N. R. Hegde, K. A. Goldsmith, D. A. Rauch, R. J. Roller, C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and D. C. Johnson. 2001. Herpes simplex virus with highly reduced gD levels can efficiently enter and spread between human keratinocytes. J. Virol. 75:10309-10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hukkanen, V., H. Mikola, M. Nykanen, and S. Syrjanen. 1999. Herpes simplex virus type 1 infection has two separate modes of spread in three-dimensional keratinocyte culture. J. Gen. Virol. 80:2149-2155. [DOI] [PubMed] [Google Scholar]
- 20.Hung, S. L., Y. Y. Cheng, Y. H. Wang, K. W. Chang, and Y. T. Chen. 2002. Expression and roles of herpesvirus entry mediators A and C in cells of oral origin. Oral Microbiol. Immunol. 17:215-223. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov, A. I., A. Nusrat, and C. A. Parkos. 2004. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell 15:176-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koyama, A. H., and T. Uchida. 1987. The mode of entry of herpes simplex virus type 1 into Vero cells. Microbiol. Immunol. 31:123-130. [DOI] [PubMed] [Google Scholar]
- 24.Lycke, E., B. Hamark, M. Johansson, A. Krotochwil, J. Lycke, and B. Svennerholm. 1988. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch. Virol. 101:87-104. [DOI] [PubMed] [Google Scholar]
- 25.Marozin, S., U. Prank, and B. Sodeik. 2004. Herpes simplex virus type 1 infection of polarized epithelial cells requires microtubules and access to receptors present at cell-cell contact sites. J. Gen. Virol. 85:775-786. [DOI] [PubMed] [Google Scholar]
- 26.Marsh, M., and A. Helenius. 1989. Virus entry into animal cells. Adv. Virus Res. 36:107-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messam, C. A., J. Hou, R. M. Gronostajski, and E. O. Major. 2003. Lineage pathway of human brain progenitor cells identified by JC virus susceptibility. Ann. Neurol. 53:636-646. [DOI] [PubMed] [Google Scholar]
- 28.Meyers, C., S. S. Andreansky, and R. J. Courtney. 2003. Replication and interaction of herpes simplex virus and human papillomavirus in differentiating host epithelial tissue. Virology 315:43-55. [DOI] [PubMed] [Google Scholar]
- 29.Miller, N., and L. M. Hutt-Fletcher. 1992. Epstein-Barr virus enters B cells and epithelial cells by different routes. J. Virol. 66:3409-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 31.Nemerow, G. R., and N. R. Cooper. 1984. Early events in the infection of human B lymphocytes by Epstein-Barr virus: the internalization process. Virology 132:186-198. [DOI] [PubMed] [Google Scholar]
- 32.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicola, A. V., and S. E. Straus. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J. Virol. 78:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15:414-422. [DOI] [PubMed] [Google Scholar]
- 35.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 37.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 38.Schelhaas, M., M. Jansen, I. Haase, and D. Knebel-Morsdorf. 2003. Herpes simplex virus type 1 exhibits a tropism for basal entry in polarized epithelial cells. J. Gen. Virol. 84:2473-2484. [DOI] [PubMed] [Google Scholar]
- 39.Sears, A. E., B. S. McGwire, and B. Roizman. 1991. Infection of polarized MDCK cells with herpes simplex virus 1: two asymmetrically distributed cell receptors interact with different viral proteins. Proc. Natl. Acad. Sci. USA 88:5087-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 41.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 42.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spinozzi, F., M. C. Pagliacci, G. Migliorati, R. Moraca, F. Grignani, C. Riccardi, and I. Nicoletti. 1994. The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells. Leuk. Res. 18:431-439. [DOI] [PubMed] [Google Scholar]
- 44.Syrjanen, S., H. Mikola, M. Nykanen, and V. Hukkanen. 1996. In vitro establishment of lytic and nonproductive infection by herpes simplex virus type 1 in three-dimensional keratinocyte culture. J. Virol. 70:6524-6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tal-Singer, R., C. Peng, M. Ponce De Leon, W. R. Abrams, B. W. Banfield, F. Tufaro, G. H. Cohen, and R. J. Eisenberg. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J. Virol. 69:4471-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topp, K. S., A. L. Rothman, and J. H. Lavail. 1997. Herpes virus infection of RPE and MDCK cells: polarity of infection. Exp. Eye Res. 64:343-354. [DOI] [PubMed] [Google Scholar]
- 47.Tran, L. C., J. M. Kissner, L. E. Westerman, and A. E. Sears. 2000. A herpes simplex virus 1 recombinant lacking the glycoprotein G coding sequences is defective in entry through apical surfaces of polarized epithelial cells in culture and in vivo. Proc. Natl. Acad. Sci. USA 97:1818-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanhaesebroeck, B., S. J. Leevers, K. Ahmadi, J. Timms, R. Katso, P. C. Driscoll, R. Woscholski, P. J. Parker, and M. D. Waterfield. 2001. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70:535-602. [DOI] [PubMed] [Google Scholar]
- 50.Visalli, R. J., R. J. Courtney, and C. Meyers. 1997. Infection and replication of herpes simplex virus type 1 in an organotypic epithelial culture system. Virology 230:236-243. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe, T., K. Kondo, and M. Oishi. 1991. Induction of in vitro differentiation of mouse erythroleukemia cells by genistein, an inhibitor of tyrosine protein kinases. Cancer Res. 51:764-768. [PubMed] [Google Scholar]
- 52.Whitley, R. J., and B. Roizman. 2001. Herpes simplex virus infections. Lancet 357:1513-1518. [DOI] [PubMed] [Google Scholar]
- 53.Wilson, D. W., N. Davis-Poynter, and A. C. Minson. 1994. Mutations in the cytoplasmic tail of herpes simplex virus glycoprotein H suppress cell fusion by a syncytial strain. J. Virol. 68:6985-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wittels, M., and P. G. Spear. 1991. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 18:271-290. [DOI] [PubMed] [Google Scholar]
- 55.Yoon, M., and P. G. Spear. 2002. Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J. Virol. 76:7203-7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zenni, M. K., P. C. Giardina, H. A. Harvey, J. Shao, M. R. Ketterer, D. M. Lubaroff, R. D. Williams, and M. A. Apicella. 2000. Macropinocytosis as a mechanism of entry into primary human urethral epithelial cells by Neisseria gonorrhoeae. Infect. Immun. 68:1696-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou, G., and B. Roizman. 2002. Cation-independent mannose 6-phosphate receptor blocks apoptosis induced by herpes simplex virus 1 mutants lacking glycoprotein D and is likely the target of antiapoptotic activity of the glycoprotein. J. Virol. 76:6197-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]