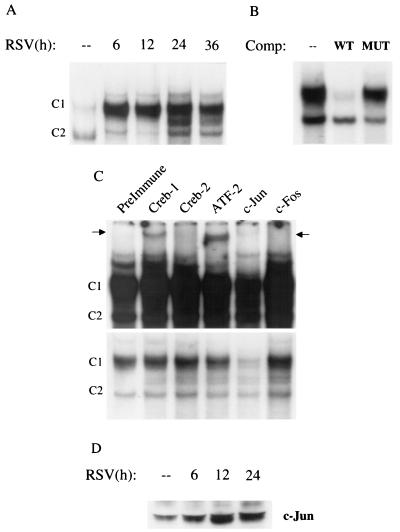
FIG. 5.
EMSA of RANTES CRE binding complexes in response to RSV infection. (A) Autoradiogram of time course. Nuclear extracts were prepared from serum-starved control and RSV-infected A549 cells at the indicated times and used for EMSA. Time following RSV infection is indicated at the top. Two DNA-protein complexes, C1 and C2, are detected in control cells. C1 binding is further increased by RSV infection, while C2 binding is not. (B) Competition (Comp) analysis. Nuclear extracts from 24-h-infected cells were used to bind to the CRE probe in the absence (–) or presence of 2 pmol of wild-type (WT) or mutated (MUT) unlabeled competitor in the binding reaction, as indicated at top. C1 is competed by the wild-type oligonucleotide but not by the mutant one, indicating binding specificity. (C) Supershift interference assay. Nuclear extracts of A549 cells infected for 12 h were used in the EMSA in the presence of preimmune serum and anti-Jun, Fos, CREB-1, -CREB-2, and -ATF-2 antibodies. Top, long exposure showing the presence of supershifted bands (indicated by the arrows) induced by the anti-CREB-1 and anti-ATF-2 antibodies; bottom, light exposure showing the disappearance of C1 induced by the anti-Jun antibody. (D) Western blot of c-Jun in A549 cells infected with RSV. A549 cells were infected with RSV (MOI of 1) for various lengths of time. Nuclear extracts were prepared from control and infected cells, and equal amounts of protein were assayed for c-Jun protein.