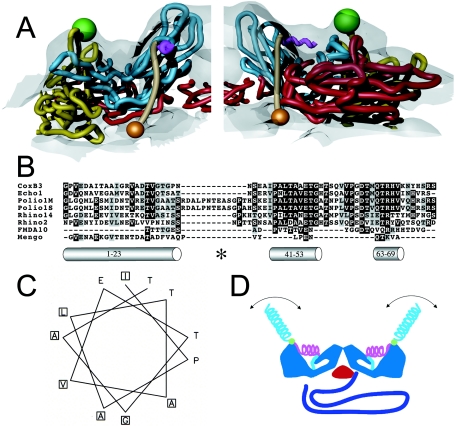
FIG. 4.
Localization and characterization of the N terminus of VP1. (A) Two views of 135S model coordinates, VP1 (cyan), VP2 (yellow), and VP3 (red), are overlaid on the cryo-EM 135S reconstruction (grey semitransparent). Trajectory for the N-terminal 70 residues of VP1 (tan) begins at residue 71 (orange sphere), emerges from a gap between fivefold symmetry-related copies of VP1, forms a helix (magenta) through the ridge, and connects to the V8-cleavage site (green sphere). The helix originates at the points of the star-shaped mesa. For clarity, only one protomer is shown in each panel. (B) Sequence alignment of the N-terminal extension of VP1 from different picornaviruses (coxsackievirus B3, echovirus 1, poliovirus 1 Mahoney strain, poliovirus 1 Sabin strain, rhinovirus 14, rhinovirus 2, foot-and-mouth-disease virus 10, and mengovirus, top to bottom, respectively) reveals a highly conserved stretch of residues (41 to 53, mostly dark highlighting) that is predicted by the Chou-Fasman algorithm to include an alpha helix. The algorithm also identified the extreme N-terminal 20 residues as a helix. This amphipathic helix was previously identified, and it was proposed to form a pore in the host cell membrane (14). A third helical stretch, located near residue 70, is observed in the native virus making contacts with the VP1 β barrel. All predicted helices are depicted as grey cylinders and labeled with their respective residue numbers. The V8 cleavage site is labeled (*). (C) A helical wheel representation (generated using EMBOSS Pepwheel, www.hgmp.mrc.ac.uk/Software/EMBOSS/Apps/pepwheel.html) of VP1 residues 41 to 52 reveals an amphipathic arrangement of side chains. (D) Schematic of the externalization of the VP1 N terminus showing the capsid (blue), VP3 β tube (red), RNA (purple), and the N terminus of VP1 (residues 71 to 54 [cyan], 53 to 41 [magenta helix], V8 cleavage site [green sphere], and 31 to 1 [cyan helix]). The N-terminal 20 residues that are thought to form an amphipathic helix (14) could be either disordered or mobile in the absence of lipid (cyan helix).