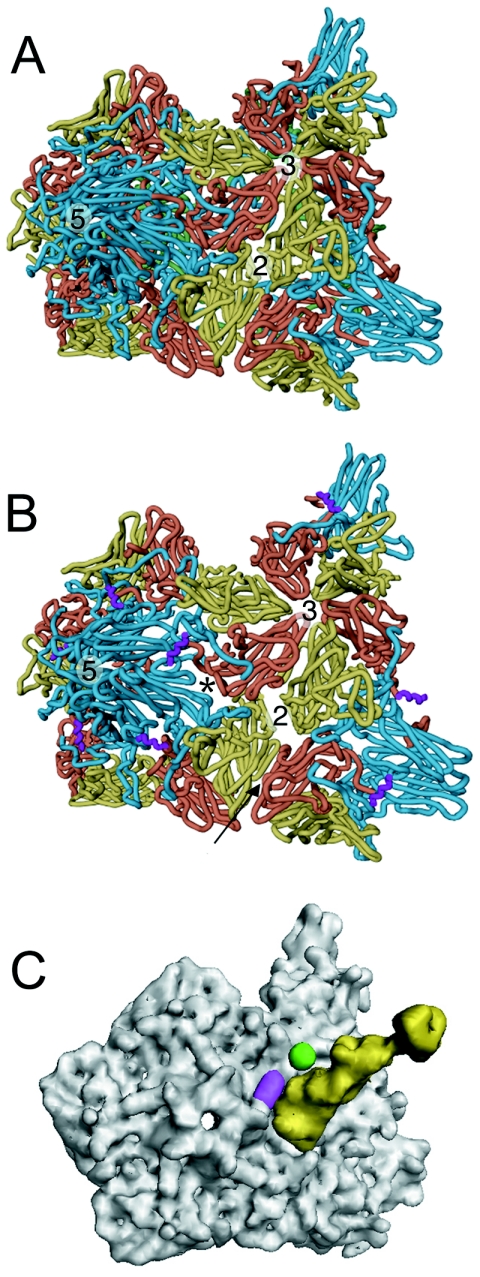
FIG. 5.
Comparison of 135S with native virus. The transition from native poliovirus (A) to the 135S intermediate (B) is characterized by an expansion of the virus capsid causing gaps in the model between symmetry-related copies of the protomer. The most pronounced openings occur between VP1 and VP3 at the base of the canyon (*) and at the twofold axis. VP1, VP2, and VP3 are cyan, yellow, and red, respectively. In the 135S model, the helix proposed to occupy the external linear ridge is magenta; in native virus, VP4 is green. The arrow indicates the six-stranded β sheet formed by the hairpin of VP2 and the four-stranded β sheet from VP3 of a different, symmetry-related pentamer. (C) Electron density calculated for Pvr (yellow) overlaid with density calculated for the pseudoatomic 135S model (grey) does not clash with the ridge corresponding to the externalized N terminus of VP1 (magenta) or with the V8 cleavage site (green).