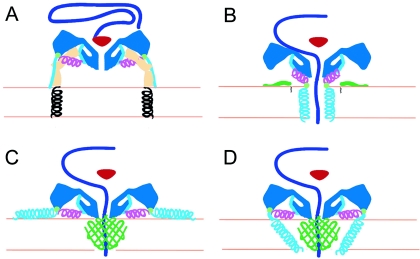
FIG. 6.
Working models for poliovirus entry. A cross section of a portion of the capsid is shown in dark blue, VP4 is green, and the N terminus of VP1 is cyan and magenta. The V8 cleavage site is shown as a green circle. (A) Native poliovirus binds its receptor, Pvr (ectodomains 1 to 3, tan; transmembrane domain, black helix), and at physiological temperature undergoes an irreversible change to the 135S particle. The path of egress of the N terminus of VP1, suggested by the present 135S reconstruction, would not preclude the continued binding of Pvr. At this stage, the VP3 β tube (red) blocks an otherwise open channel along the fivefold axis. (B to D) Alternative models for the direct anchoring of the virus to the membrane via the N terminus of VP1 and formation of a transmembrane pore for RNA translocation. To accommodate the passage of RNA (purple), the VP3 β tube has shifted, and the channel has expanded, becoming continuous with a pore through the membrane. The V8 cleavage site remains accessible. (B) Amphipathic helices at the N terminus of VP1 (cyan) may form a five-helix bundle close to the fivefold axis, which would require the magenta helix to dissociate from the body of the virus. Alternatively, VP4 may play a more central role in pore formation (C and D). In that case, VP1 may participate directly in forming the pore (D) or serve as a nonspecific membrane anchor (C).