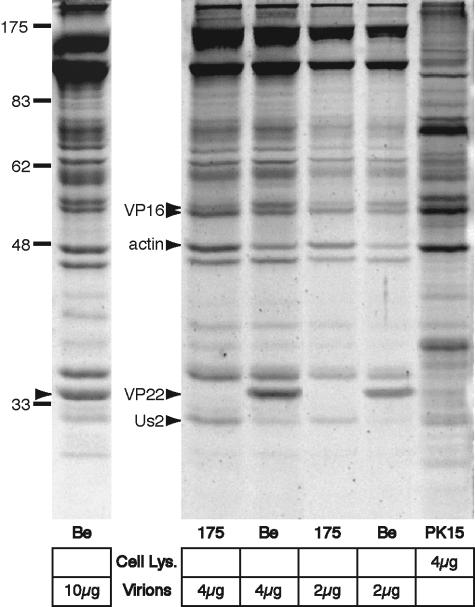
FIG. 1.
The compensating pool of tegument proteins in PRV VP22-null virions is specific and includes cellular actin. Wild-type PRV Becker (Be) and VP22-null (PRV 175) virions were purified by centrifugation on a linear tartrate gradient (5 to 20%), and the indicated amounts were separated by SDS-PAGE. Total protein was stained using Sypro Ruby Red and detected by UV illumination. The PRV VP22 homologue was identified as a 34-kDa virion protein and a major component of Be virions (10 μg, left arrowhead). The levels of three proteins increased in VP22-null (PRV 175) virions in comparison to wild type (Be). Two of these proteins, 28 and 46 kDa in size, were excised and identified using mass spectrometry as the PRV Us2 tegument protein and cellular actin, respectively (right arrowheads). Cellular actin was confirmed to be a major component of extracellular virions. A 53-kDa protein also increased in VP22-null virions and was identified as the tegument protein VP16 (arrowhead). The migration positions of molecular mass markers are shown in kilodaltons.