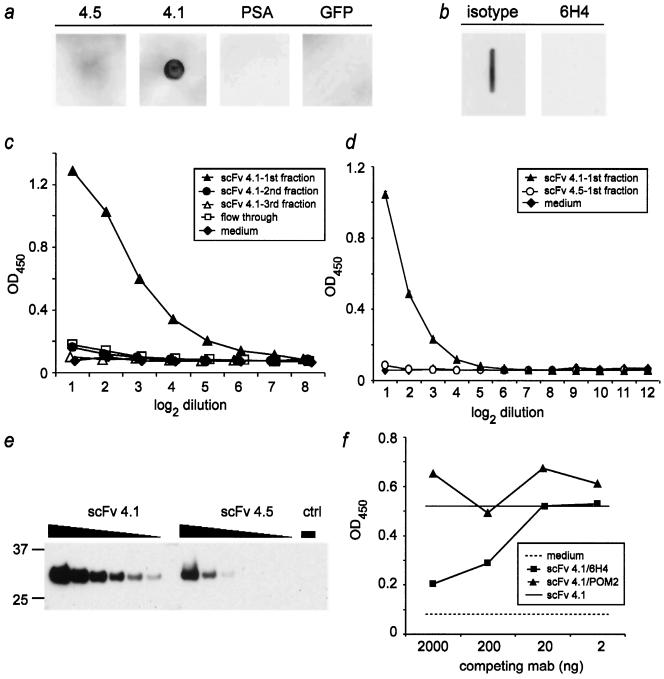
FIG. 2.
Specificity and PrP binding properties of scFv fragments. a: Recombinant PrP (PrPREC; 200 ng) was spotted onto nitrocellulose and incubated with medium derived from RD-4 cells stably expressing anti-PrP scFv (clone 4.5 and clone 4.1), GFP, and PSA. Only supernatant derived from RD-4 cells transfected with anti-PrP scFv clone 4.1 recognized PrPREC. b: monoclonal antibody 6H4, but not the IgG isotype control, antagonized binding to PrPREC when administered prior to incubation with sc4.1. c, d: Affinity-purified sc4.1 (filled triangles) resulted in concentration-dependent binding to PrPREC as assessed by ELISA. In contrast, none of the negative controls or sc4.5 (d) bound PrPC. e: The relative concentrations of sc4.1 and sc4.5 in the preparations used in panels c and d were estimated by probing serially twofold diluted scFvs with an antibody to the myc tag upon Western blotting. The negative control (ctrl) was Ni-NTA-purified medium of nontransfected HEK-293 cells. Numbers on the left indicate molecular masses in kDa. f: Pretreatment of PrPREC-coated ELISA plates with increasing amounts of monoclonal antibody 6H4 (recognizing mouse PrP144-152) inhibited binding of 4 μg purified scFvs (clone 4.1) to PrPREC (squares), while anti-PrP antibody POM2 (which recognizes an amino-terminal epitope of PrPC) did not alter PrP reactivity of sc 4.1 (triangles). Solid line, binding of scFvs 4.1 to PrPREC in the absence of competing antibodies; dashed line, background as determined by the unspecific binding of medium to PrPREC. In panels c, d, and f, each symbol represents the mean of duplicate measurements, except in panel d, where the mean and standard deviation of triplicates are depicted. Abscissa, twofold serial dilutions of scFvs (c and d) or amount of competing monoclonal antibody (mab) per well (in ng) (f). Ordinate, optical density at 450 nm.