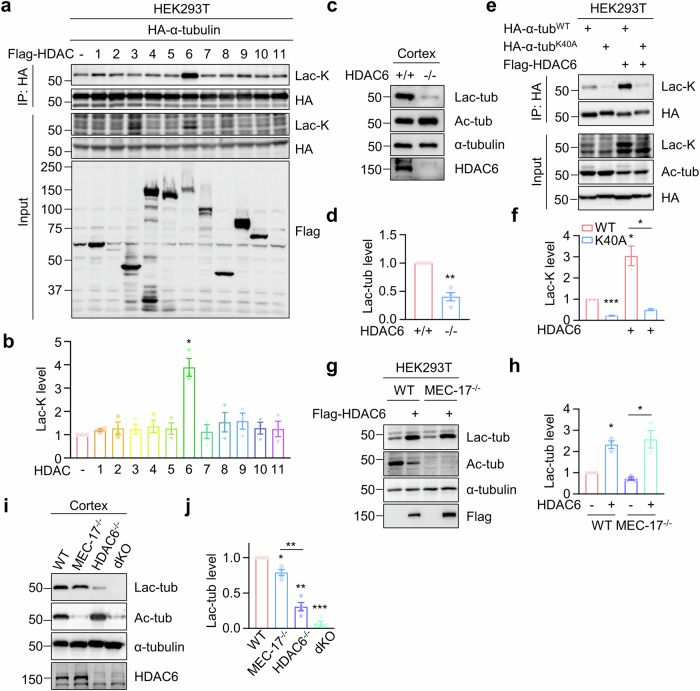
Fig. 2. Identification of HDAC6 as a primary lactyltransferase for α-tubulin lactylation.
a, b HDAC6 overexpression leads to increased α-tubulin lactylation. HEK293T cells were transfected with Flag-tagged HDAC family proteins, together with HA-α-tubulin. HA-α-tubulin was immunoprecipitated with anti-HA antibody and α-tubulin lactylation was revealed by anti-Lac-K antibody. n = 3 experiments. Two-sided paired student’s t-test, HDAC6 vs control, p = 0.0170. c, d α-Tubulin lactylation is largely reduced in HDAC6-deficient cortex. n = 4 experiments. Two-sided paired student’s t-test, HDAC6-/- vs HDAC6+/+, p = 0.0038. e, f HDAC6-induced α-tubulin lactylation occurs on K40 residue. n = 3 experiments. Two-sided paired student’s t-test, K40A vs WT, p = 0.0001; WT + HDAC6 vs WT, p = 0.0473; K40A + HDAC6 vs WT + HDAC6, p = 0.0369. g, h HDAC6-induced α-tubulin lactylation is independent of α-tubulin acetylation. n = 3 experiments. Two-sided paired student’s t-test, WT + HDAC6 vs WT, p = 0.0171; MEC-17-/- + HDAC6 vs MEC-17-/-, p = 0.0326. i, j HDAC6 plays the primary role in regulating α-tubulin lactylation. n = 4 experiments. Two-sided paired student’s t-test, MEC-17-/- vs WT, p = 0.0146; HDAC6-/- vs WT, p = 0.0014; dKO vs WT, p = 0.0001; HDAC6-/- vs MEC-17-/-, p = 0.0055. Data are shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Source data are provided as a Source Data file.