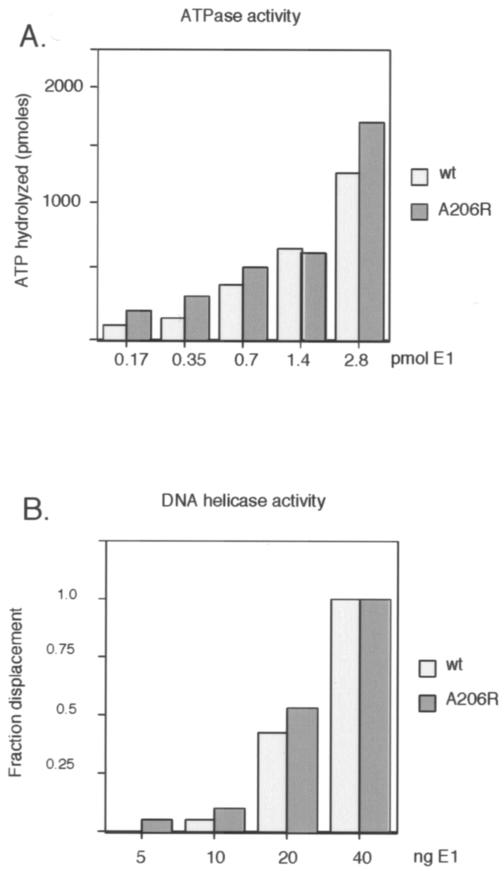
FIG. 2.
The dimerization defect of E1 A206R does not affect the ATPase and DNA helicase activities of the E1 protein. (A) Five quantities of wt and mutant E1 (0.17, 0.35, 0.7, 1.4, and 2.8 pmol) were compared for the ability to hydrolyze ATP in the presence of ssDNA essentially as described previously (4). The reaction products were analyzed by thin-layer chromatography, and the quantity of hydrolyzed ATP was determined. (B) E1 A206R and wt E1 proteins have similar DNA helicase activities. The DNA helicase activity of E1 A206R and wt E1 were compared using a partially double-stranded M13 template as a substrate, essentially as described previously (14). A 50-mer oligonucleotide with 28 bp of complementarity to M13 was end labeled and annealed to M13 DNA, generating a substrate with a 22-nucleotide 3′ tail. Four concentrations of E1 (5, 10, 20, and 40 ng) were incubated with the substrate, and the reaction was terminated by the addition of sodium dodecyl sulfate to 0.1%. The samples were analyzed on agarose gels, and the fraction of displaced oligonucleotide was determined.