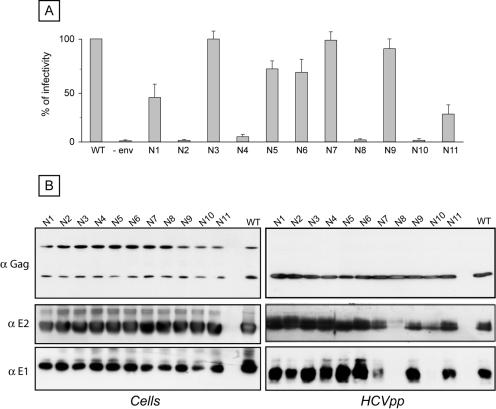
FIG. 5.
Role of E2 glycans in HCVpp infectivity. (A) Plasmids encoding E2 mutants in the context of an E1E2 polyprotein were used to generate HCVpp (E1N1 to E1N11). Control experiments in the absence of HCV envelope proteins were also performed (−env). Infection assays with the luciferase reporter gene were performed by using target Huh-7 human hepatocarcinoma cells. Similar inputs of viral particles were used in each experiment, and this was confirmed by comparing the amounts of capsid protein incorporated into HCVpp (see panel B, anti-Gag). The results are expressed as percentages of infectivity. For each mutant, the percentage of infectivity was calculated as the luciferase activity of HCVpp produced with mutant envelope proteins divided by the luciferase activity of HCVpp produced with wild-type E1 and E2 (WT). The results are reported as means ± standard deviations of six independent experiments. Pseudotyped particles produced in the absence of envelope proteins were used as a control. The mean luciferase activity of such pseudotyped particles produced in the absence of envelope proteins represented <2% of the activity measured for HCVpp. The luciferase activities (in relative light units) in a representative experiment were as follows: WT, 4.7 × 105; N1, 2.3 × 105; N2, 1.4 × 103; N3, 4.6 × 105; N4, 1.9 × 104; N5, 2.5 × 105; N6, 2.7 × 105; N7, 4.7 × 105; N8, 4.3 × 103; N9, 4.7 × 105; N10, 1.4 × 103; and N11, 1.3 × 105. (B) Incorporation of HCV envelope proteins into HCVpp. Particles were pelleted through 30% sucrose cushions and analyzed by Western blotting. HCV envelope glycoproteins and the capsid protein of MLV were revealed with the following specific MAbs: anti-E1 (A4), anti-E2 (3/11), and anti-MLV capsid (R187, anti-Gag). The expression of mutant proteins was verified by direct Western blotting of cell lysates.