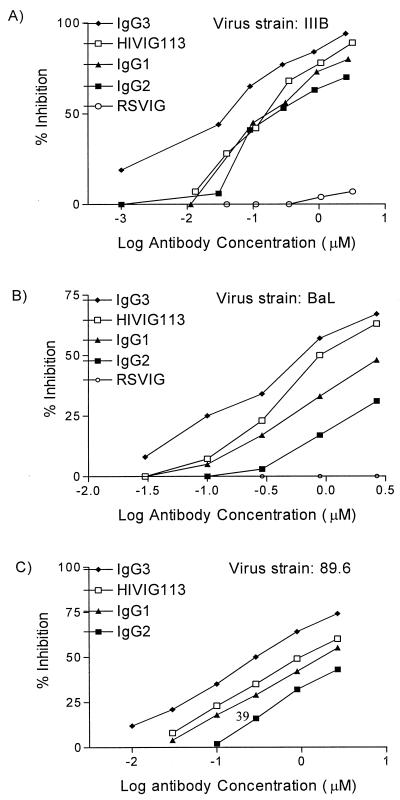
FIG. 3.
Inhibition of HIV-1 envelope-mediated fusion by HIVIG and HIVIG subclasses. Serial dilutions of antibody preparations were added to envelope-expressing cells for 1 h prior to addition of PM1 target cells. Fusion was assessed 3 to 6 h later. Fusion inhibition was compared to that of a control culture without IgG additives, which defined 0% inhibition. RSVIG was used as a negative control. Results are expressed as micromolar concentrations to account for the slightly greater molecular weight of IgG3. (A) HIV IIIB (X4) envelope-mediated fusion inhibition using TF228 cells. The ID50 (micromolar) were as follows: HIVIG, 0.18; IgG1, 0.21; IgG2, 0.37; IgG3, 0.06. (B) HIV-1 BaL (R5) envelope-mediated fusion inhibition using vCB28-infected 12E1 cells. The ID50 (micromolar) were as follows: HIVIG, 1.2; IgG1, 4.0; IgG2, 73.2; IgG3, 0.7. (C) HIV-1 89.6 (X4R5)-mediated envelope fusion inhibition using vaccinia virus strain 89.6-infected 121E1 cells. The ID50 (micromolar) were as follows: HIVIG, 1.2; IgG1, 1.75; IgG2, 4.2; IgG3, 0.6. The experimental results shown are representative of at least two fusion inhibition assays for each envelope.