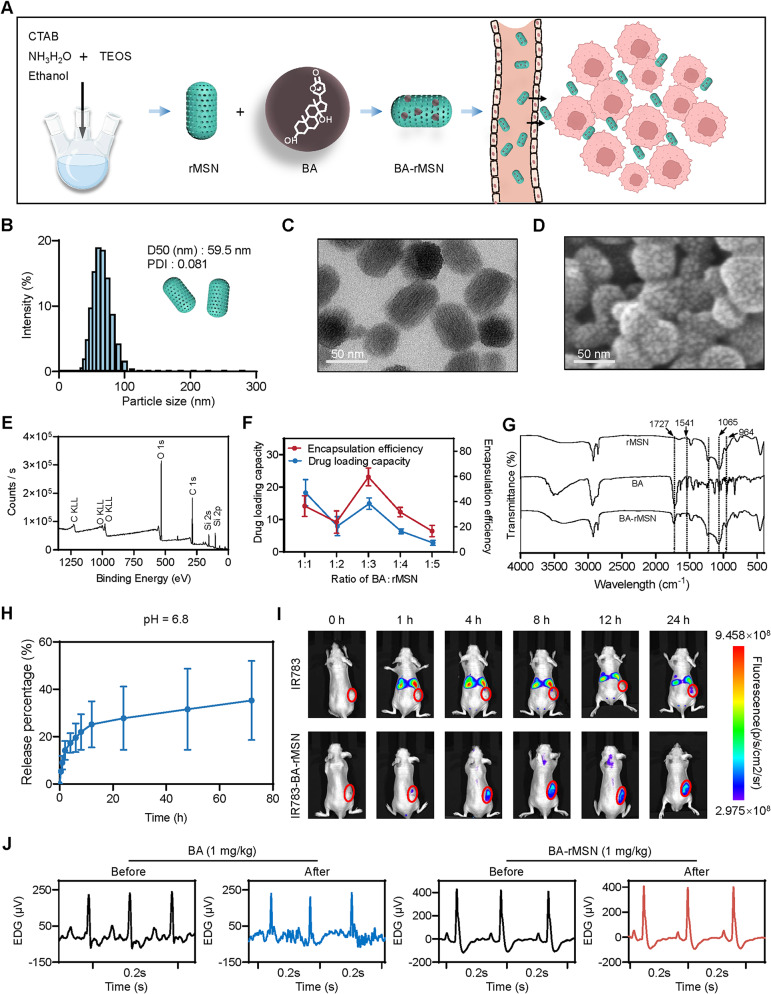
Fig. 1.
Synthesis and characterization of BA-rMSNs. (A) CTAB, NH3H2O, and TEOS were used to synthesize rMSNs; subsequently, BA was loaded into the rMSNs. (B) The median diameter (D50) of the BA-rMSNs was 59.5 nm, and the polydispersity index (PDI) was 0.081, indicating that the rMSNs had a uniform particle size distribution. (C) TEM demonstrated that the BA-rMSNs had a rod-shaped morphology. (D) SEM image showing a rod-shaped morphology with porosity. (E) XPS spectrum analysis demonstrated the elemental composition of the rMSN. (F) The drug loading capacity and encapsulation efficiency indicated that 1:3 was the optimal ratio of BA to rMSN. (G) FTIR spectroscopy was used to analyze the chemical bonds and functional groups in the rMSN, BA, and BA-rMSN. (H) The drug release rate of BA-rMSNs was measured in pH 6.8 PBS. (I) In vivo, fluorescence images proved that the rMSN enhanced the tumor-targeting ability of BA. (J) An electrocardiogram revealed that BA caused cardiotoxicity, whereas rMSNs decreased BA-mediated toxicity