Abstract
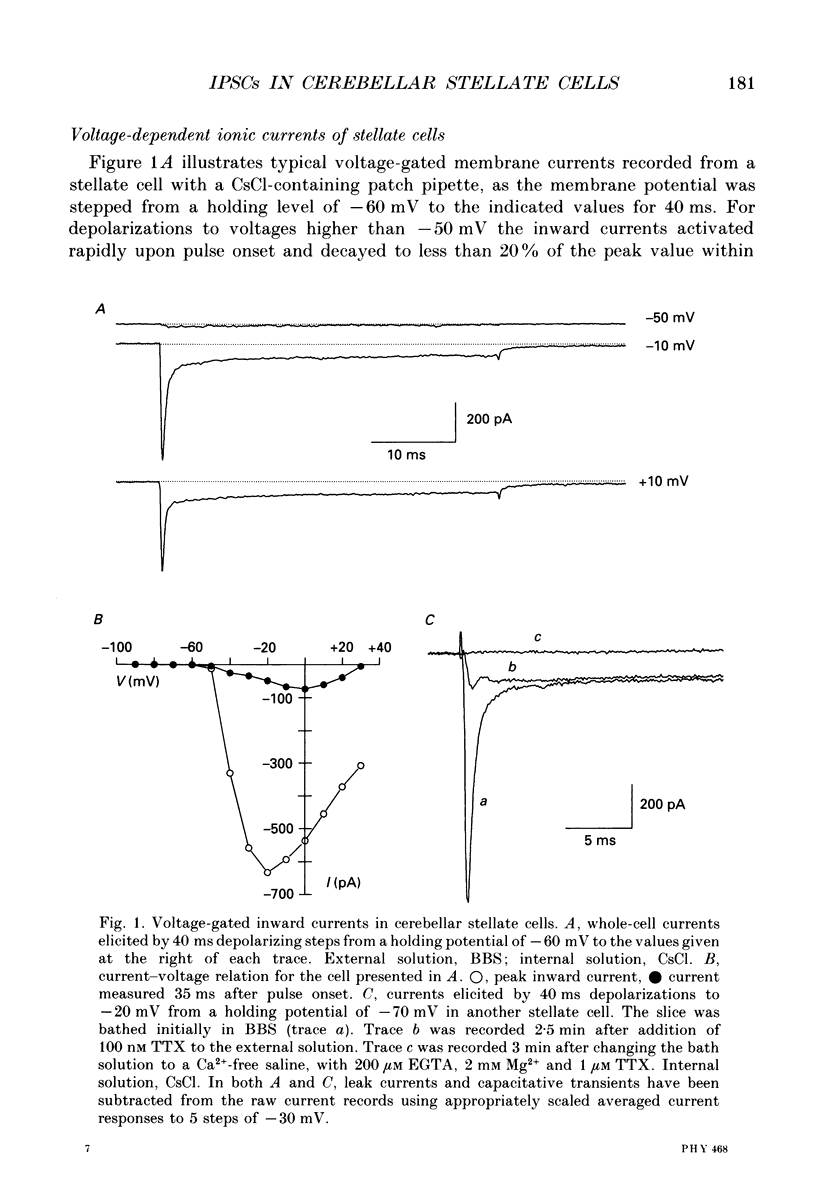
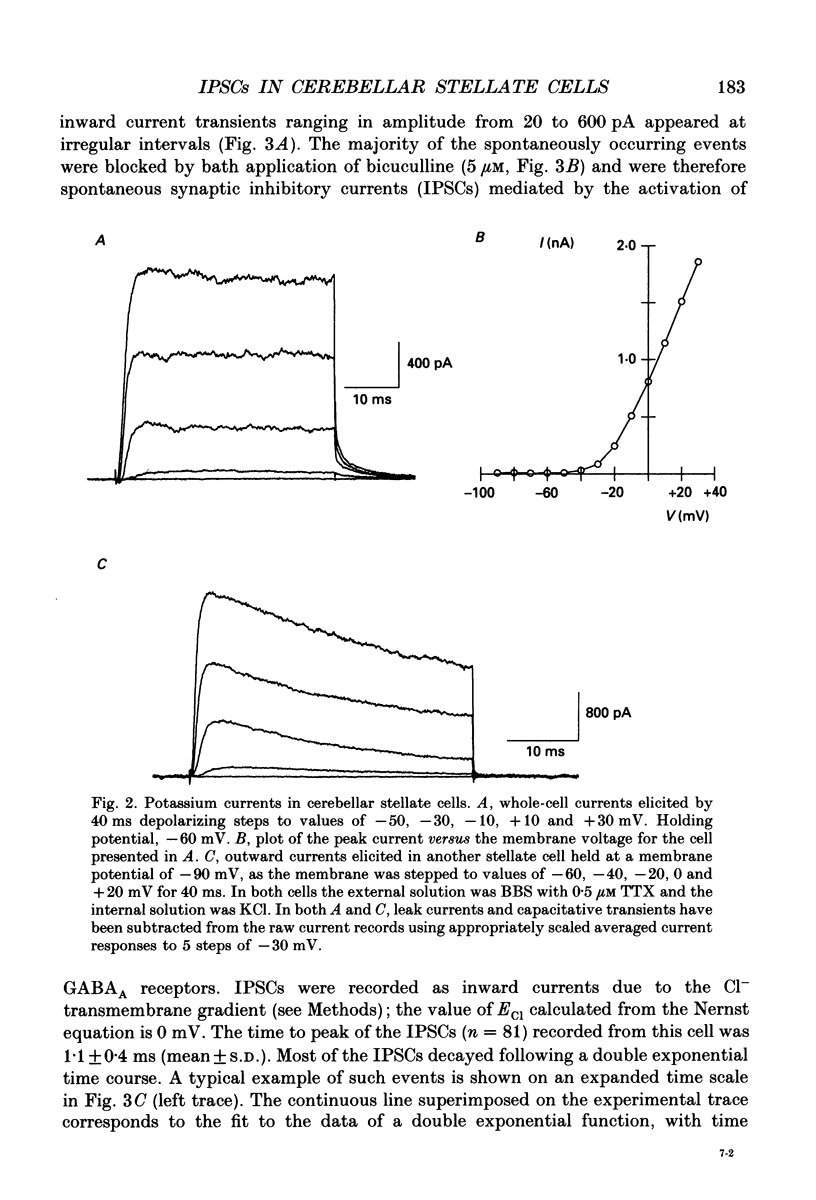
1. In thin cerebellar slices of rats aged 14-21 days, voltage-gated currents, synaptic currents and GABA responses were studied with the tight-seal whole-cell recording technique from stellate cells (8-9 micrograms soma diameter) located in the outer two-thirds of the molecular layer. 2. In symmetrical Cl- conditions, stellate cells voltage-clamped at -60 mV showed spontaneous inhibitory postsynaptic currents (IPSCs). As were the GABAA responses of the same cells, the IPSCs were blocked by bicuculline. The frequency of occurrence of IPSCs ranged from 0.2 to 1.9 events per second (21 cells). The mean amplitude of the events ranged from 61 to 226 pA (mean +/- S.E.M.: 132 +/- 11; n = 21). 3. The temporal course of IPSCs was characterized by a rapid rise (mean +/- S.E.M. of the time to peak: 1.1 +/- 0.1 ms, n = 7) and a slow decay. The decay phase was described by a double exponential function with time constants of 8.7 +/- 0.6 ms, and 40.9 +/- 3.7 ms respectively (means +/- S.E.M.; n = 7). 4. A minor fraction (15 to 20%) of the spontaneous synaptic events recorded in control saline had a faster onset than that of the IPSCs and decayed with a rapid mono-exponential decay (time constant of 1.0-1.3 ms). These were excitatory postsynaptic currents (EPSCs) unaffected by bicuculline and blocked by the glutamate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). 5. Bath application of TTX (0.5-1 microM), which blocked voltage-gated Na+ currents in stellate cells, induced a variable decrease in the frequency of IPSCs (mean +/- S.E.M. of the frequency ratio in TTX over control: 0.47 +/- 0.09; n = 12). However, the toxin had no significant effect either on the mean amplitude or on the kinetics of the IPSCs. The mean amplitude of the miniature IPSCs was 141 +/- 13 pA (mean +/- S.E.M.; n = 22). 6. In TTX-containing solutions, the frequency of the IPSCs was unaffected when Ca2+ currents were eliminated either by removal of extracellular Ca2+ and addition of EGTA, or by addition of Cd2+. Miniature IPSCs of 200-300 pA were still observed. 7. In symmetrical Cl- conditions, local application of GABA to stellate cells induced an inward current and an increase in membrane noise. Responses to prolonged applications of GABA showed desensitization in both whole-cell mode and somatic outside-out patches. The chord conductance estimated from recording single GABA channel events in somatic outside-out patches was 28 pS.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
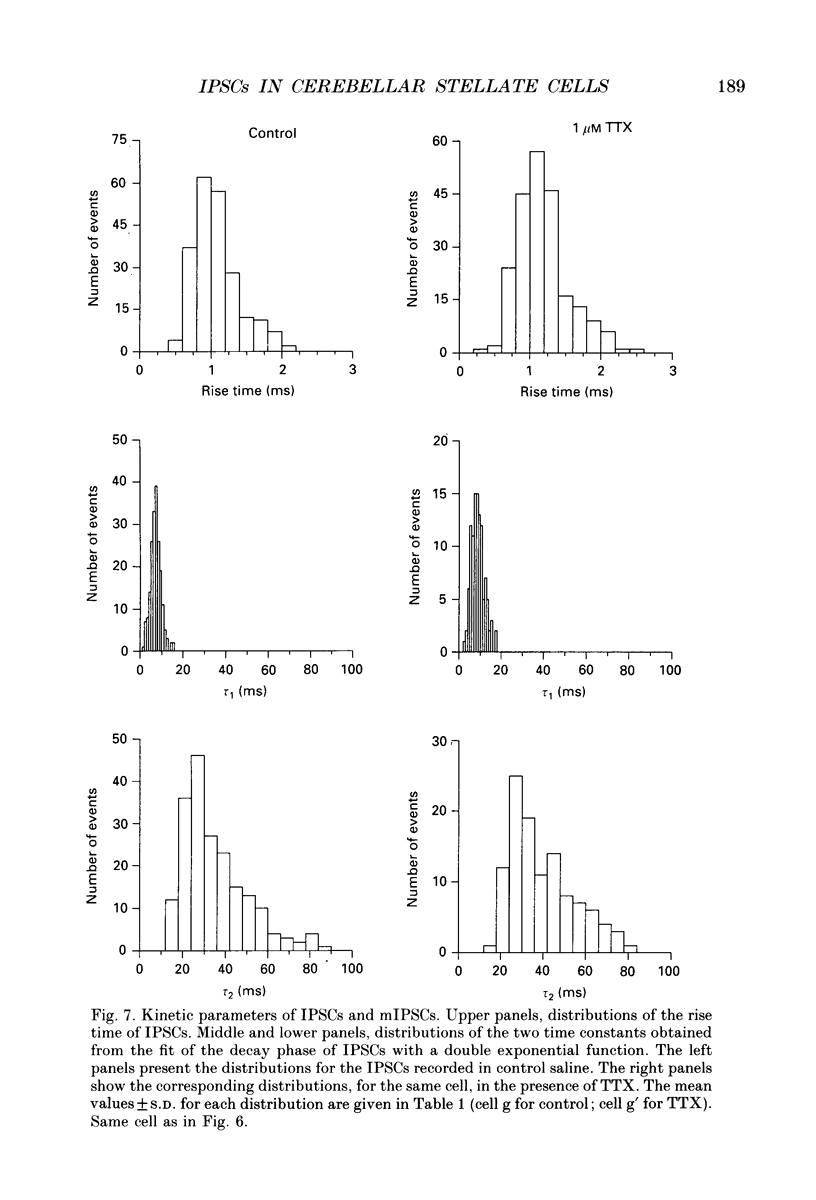
PDF




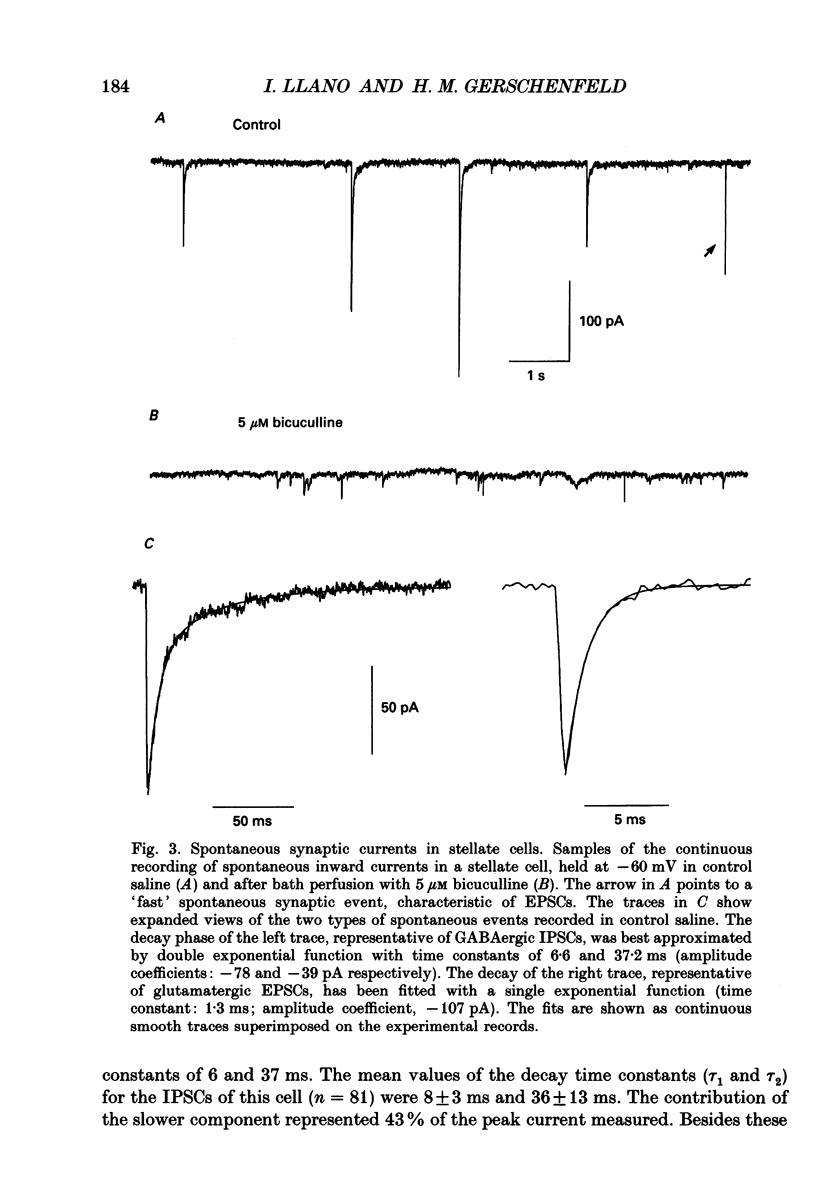


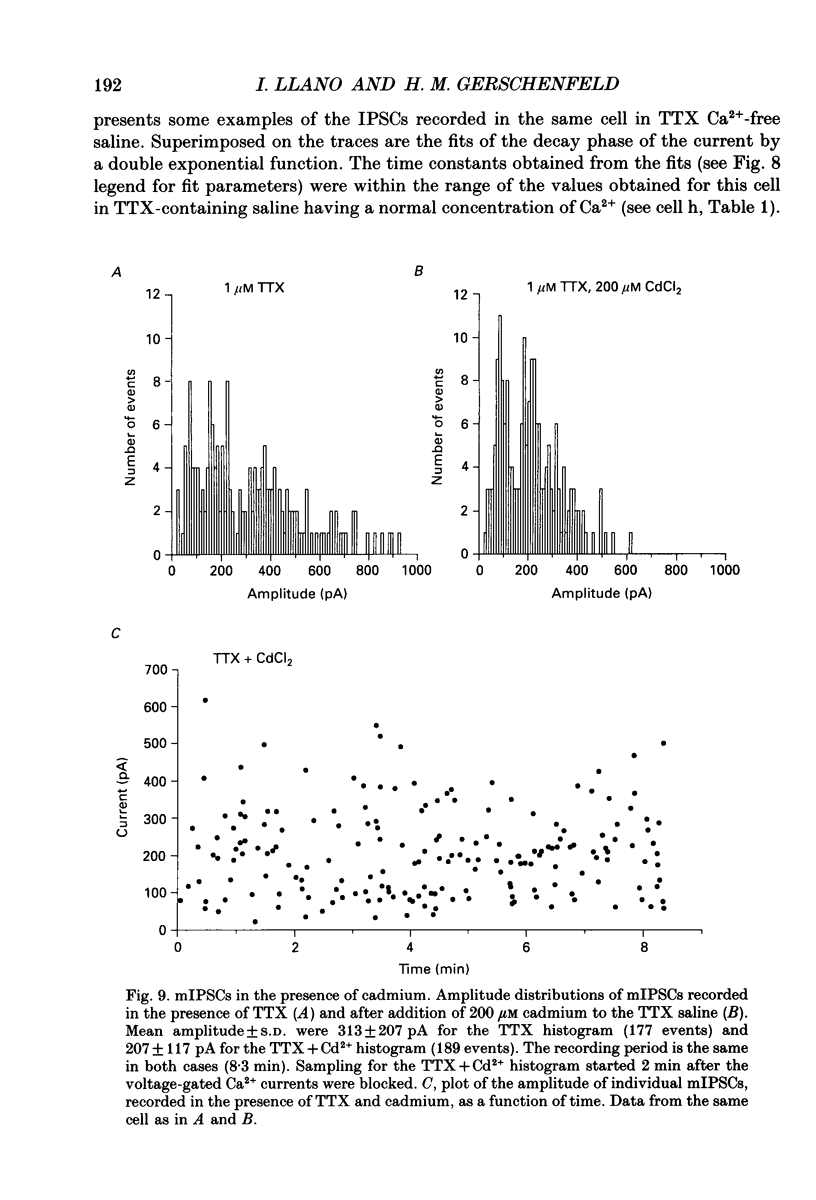






Selected References
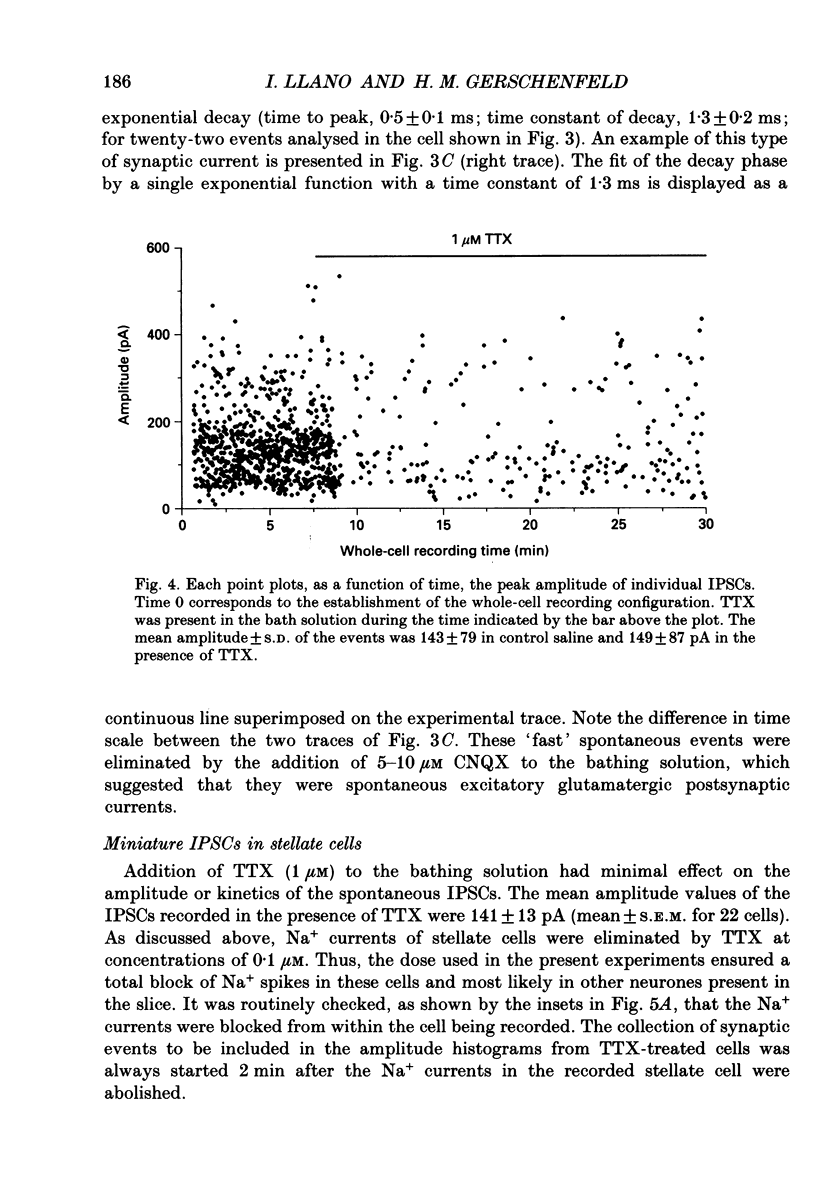
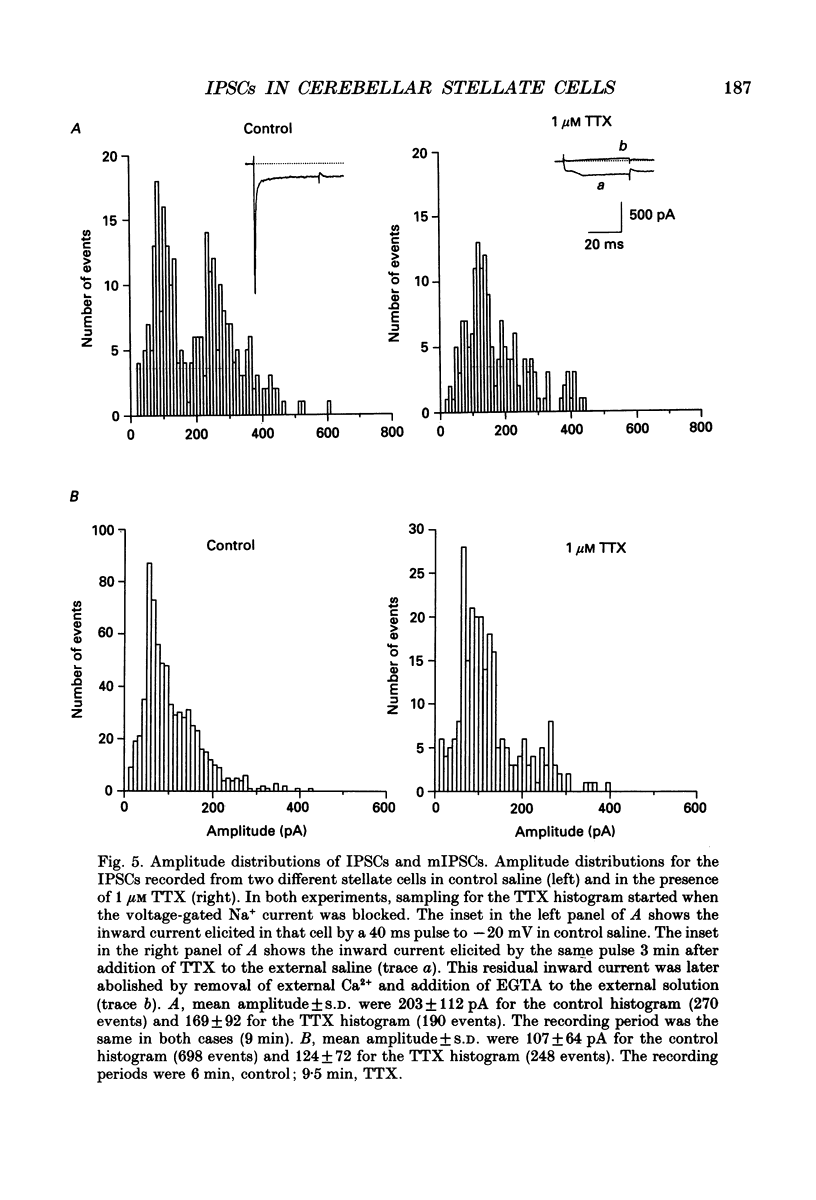
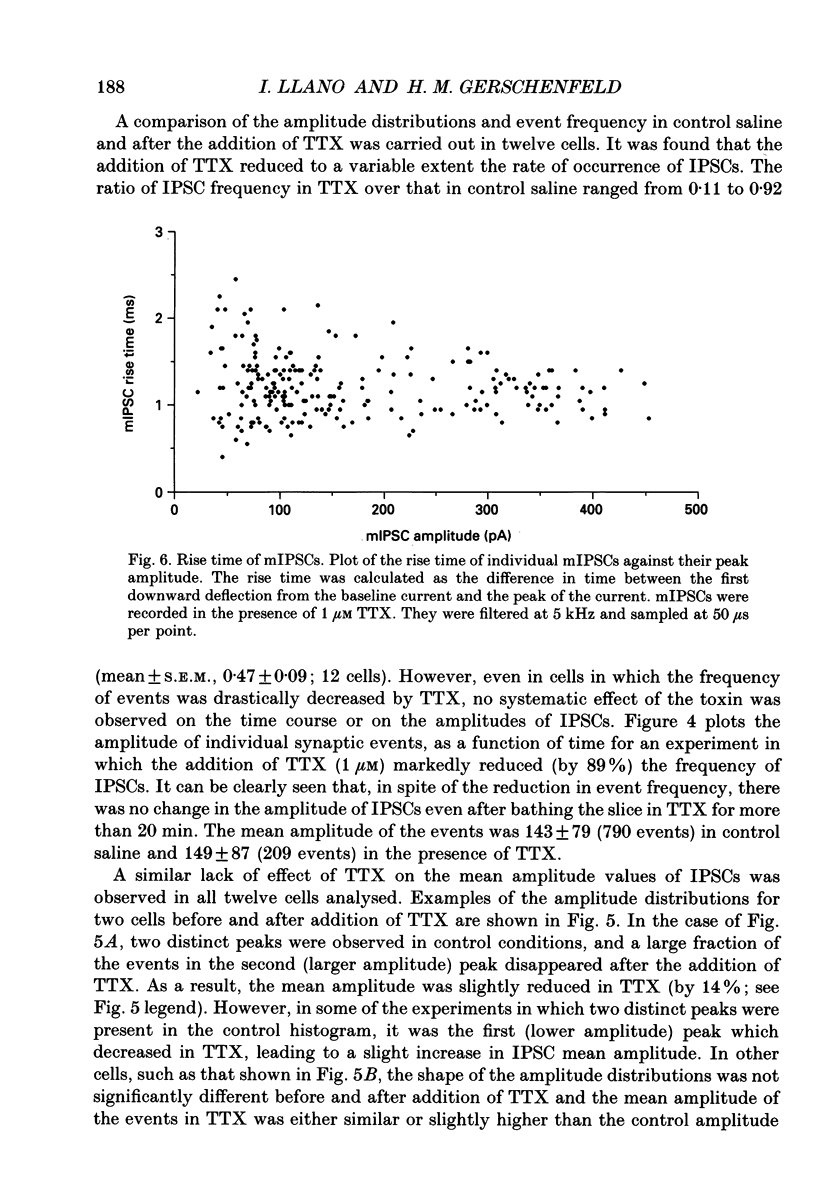
These references are in PubMed. This may not be the complete list of references from this article.
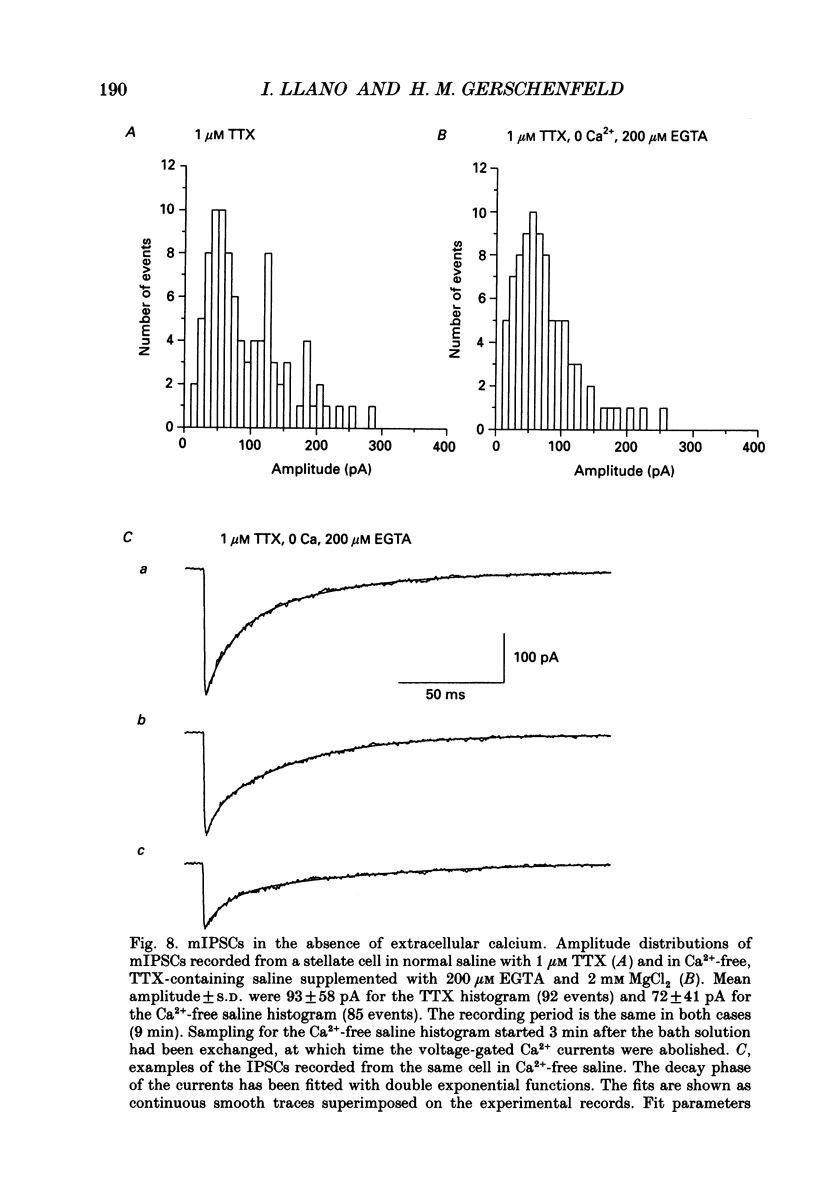
- Altman J. Experimental reorganization of the cerebellar cortex. VII. Effects of late x-irradiation schedules that interfere with cell acquisition after stellate cells are formed. J Comp Neurol. 1976 Jan 1;165(1):65–75. doi: 10.1002/cne.901650106. [DOI] [PubMed] [Google Scholar]
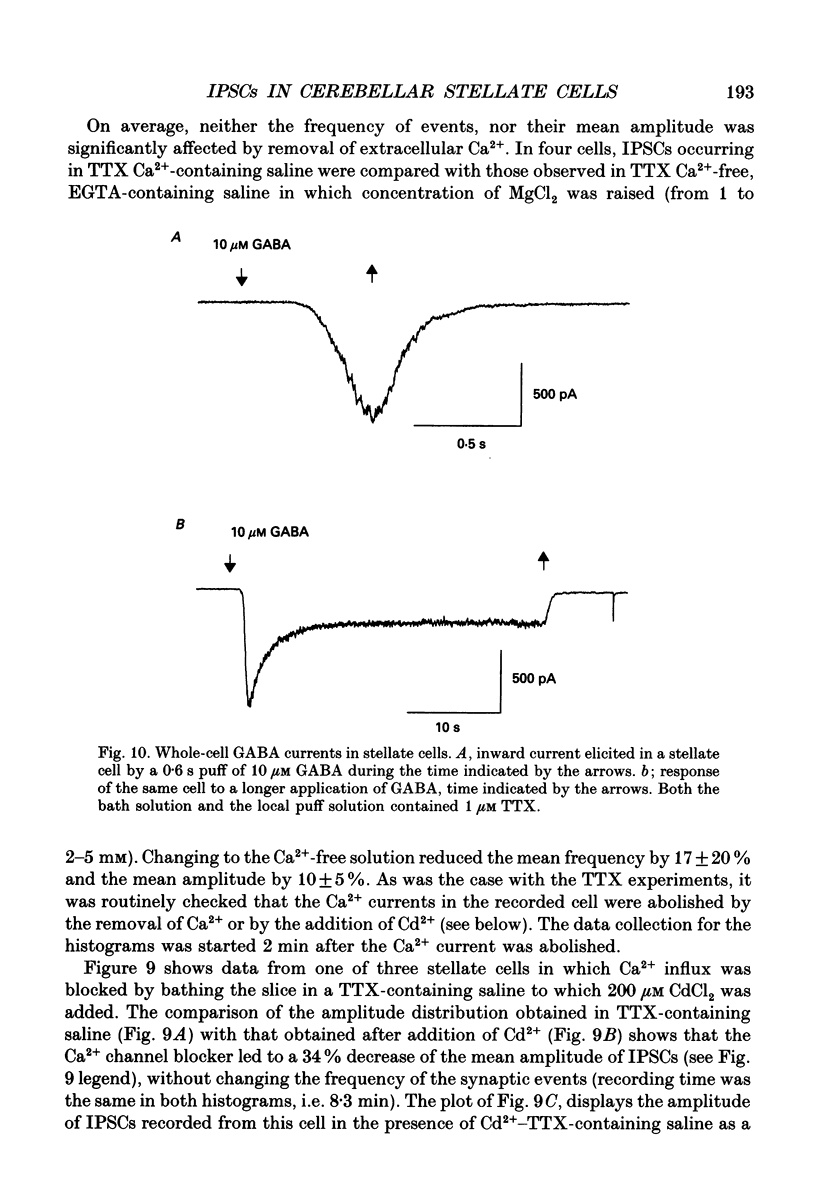
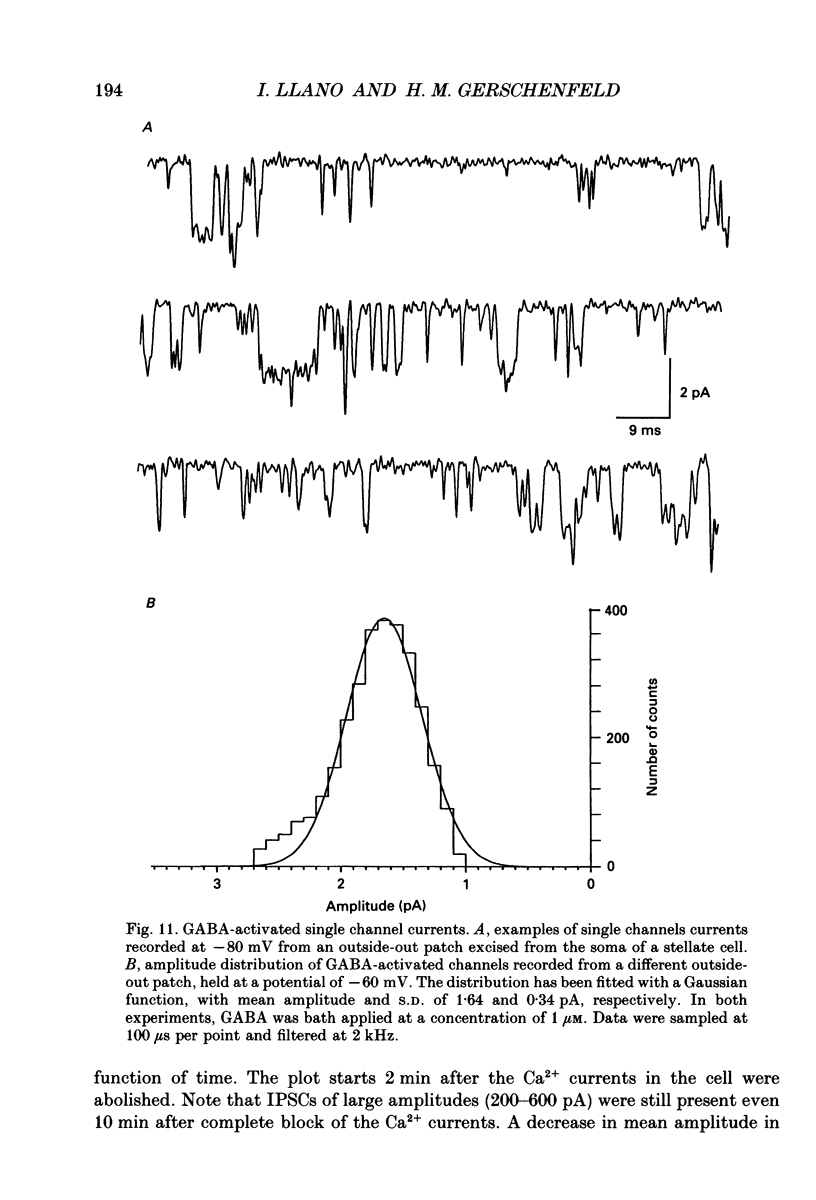
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972 Jul;145(3):353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972 Aug;145(4):399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channels in vertebrate glia. Annu Rev Neurosci. 1990;13:441–474. doi: 10.1146/annurev.ne.13.030190.002301. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L. The stellate cells of the rat's cerebellar cortex. Z Anat Entwicklungsgesch. 1972;136(2):224–248. doi: 10.1007/BF00519180. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The inhibitory interneurones within the cerebellar cortex. Exp Brain Res. 1966;1(1):1–16. doi: 10.1007/BF00235206. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A. Autoradiographic identification of cerebral and cerebellar cortical neurons accumulating labeled gamma-aminobutyric acid ( 3 H-GABA). Exp Brain Res. 1972;14(4):354–362. doi: 10.1007/BF00235032. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Blocking effects of cobalt and related ions on the gamma-aminobutyric acid-induced current in turtle retinal cones. J Physiol. 1986 Apr;373:463–479. doi: 10.1113/jphysiol.1986.sp016058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H., Faber D. S. Quantal analysis and synaptic efficacy in the CNS. Trends Neurosci. 1991 Oct;14(10):439–445. doi: 10.1016/0166-2236(91)90042-s. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Lemkey-Johnston N., Larramendi L. M. Types and distribution of synapses upon basket and stellate cells of the mouse cerebellum: an electron microscopic study. J Comp Neurol. 1968 Sep;134(1):73–112. doi: 10.1002/cne.901340106. [DOI] [PubMed] [Google Scholar]
- Llano I., Gerschenfeld H. M. Beta-adrenergic enhancement of inhibitory synaptic activity in rat cerebellar stellate and Purkinje cells. J Physiol. 1993 Aug;468:201–224. doi: 10.1113/jphysiol.1993.sp019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Leresche N., Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991 Apr;6(4):565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R. Functional aspects of interneuronal evolution in the cerebellar cortex. UCLA Forum Med Sci. 1969;11:329–348. [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T., Renner P., Nicoll R. A. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature. 1992 Jan 2;355(6355):50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- O'Donoghue D. L., King J. S., Bishop G. A. Physiological and anatomical studies of the interactions between Purkinje cells and basket cells in the cat's cerebellar cortex: evidence for a unitary relationship. J Neurosci. 1989 Jun;9(6):2141–2150. doi: 10.1523/JNEUROSCI.09-06-02141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M., Magyar P., Szentágothai J. Quantitative histological analysis of the cerebellar cortex in the cat. 3. Structural organization of the molecular layer. Brain Res. 1971 Nov;34(1):1–18. doi: 10.1016/0006-8993(71)90347-7. [DOI] [PubMed] [Google Scholar]
- Raastad Morten, Storm Johan F., Andersen Per. Putative Single Quantum and Single Fibre Excitatory Postsynaptic Currents Show Similar Amplitude Range and Variability in Rat Hippocampal Slices. Eur J Neurosci. 1992 Oct;4(1):113–117. doi: 10.1111/j.1460-9568.1992.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Ropert N., Miles R., Korn H. Characteristics of miniature inhibitory postsynaptic currents in CA1 pyramidal neurones of rat hippocampus. J Physiol. 1990 Sep;428:707–722. doi: 10.1113/jphysiol.1990.sp018236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R., López-Barneo J., Konnerth A. Excitatory and inhibitory synaptic currents and receptors in rat medial septal neurones. J Physiol. 1992 Jan;445:261–276. doi: 10.1113/jphysiol.1992.sp018923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon F., Iversen L. L. Selective accumulation of ( 3 H)GABA by stellate cells in rat cerebellar cortex in vivo. Brain Res. 1972 Jul 20;42(2):503–507. doi: 10.1016/0006-8993(72)90550-1. [DOI] [PubMed] [Google Scholar]
- Silver R. A., Traynelis S. F., Cull-Candy S. G. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992 Jan 9;355(6356):163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Vincent P., Armstrong C. M., Marty A. Inhibitory synaptic currents in rat cerebellar Purkinje cells: modulation by postsynaptic depolarization. J Physiol. 1992 Oct;456:453–471. doi: 10.1113/jphysiol.1992.sp019346. [DOI] [PMC free article] [PubMed] [Google Scholar]


