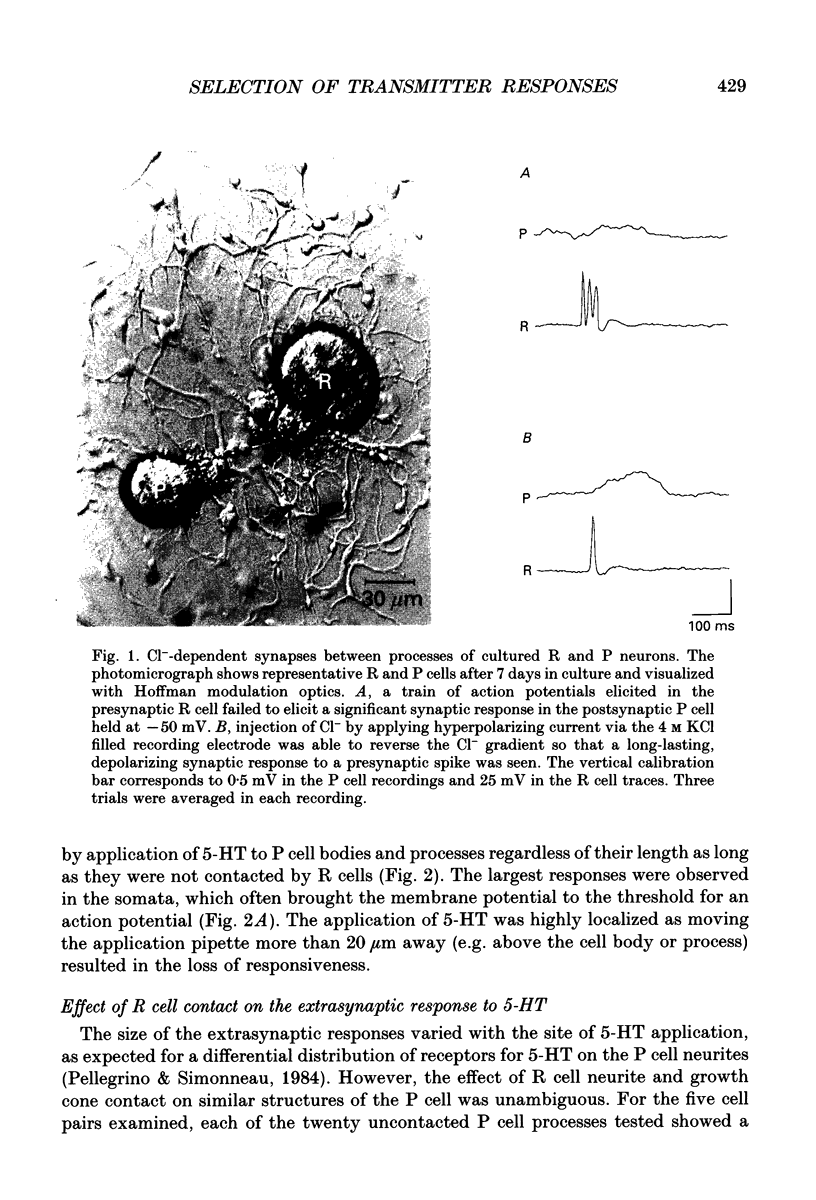
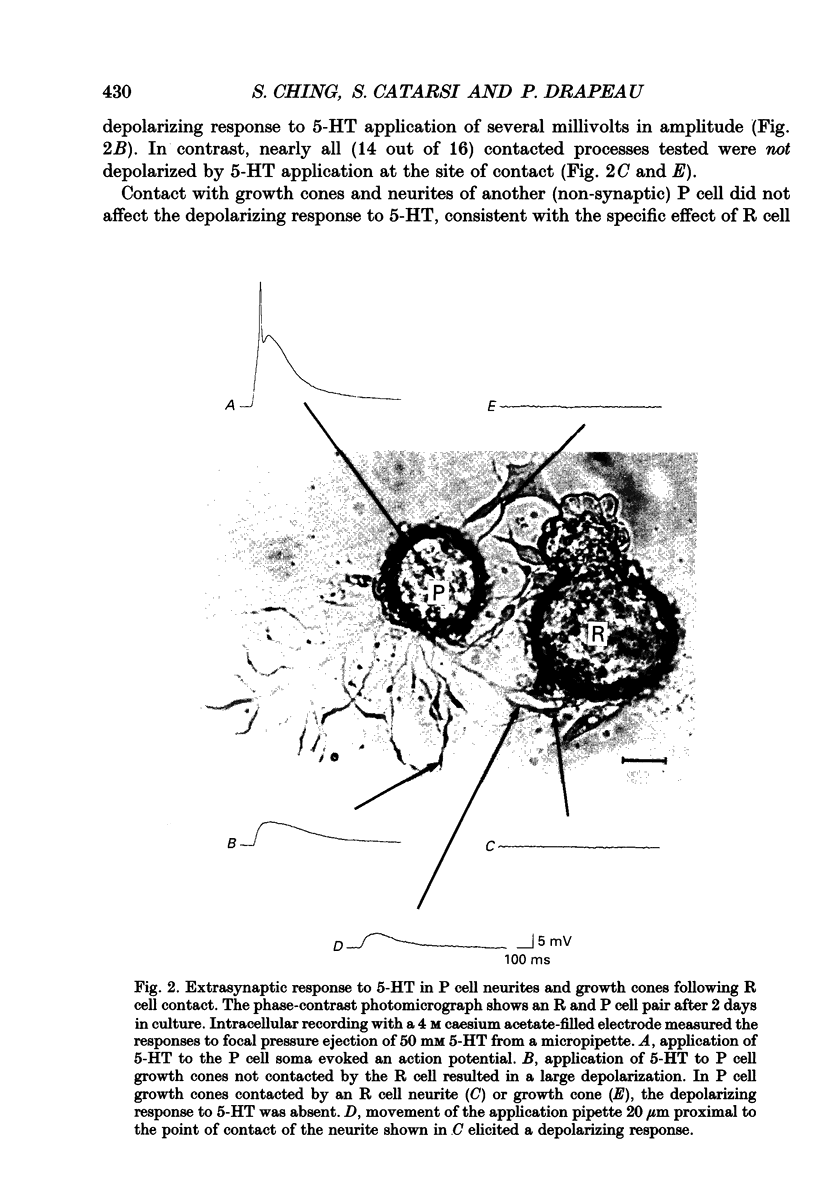
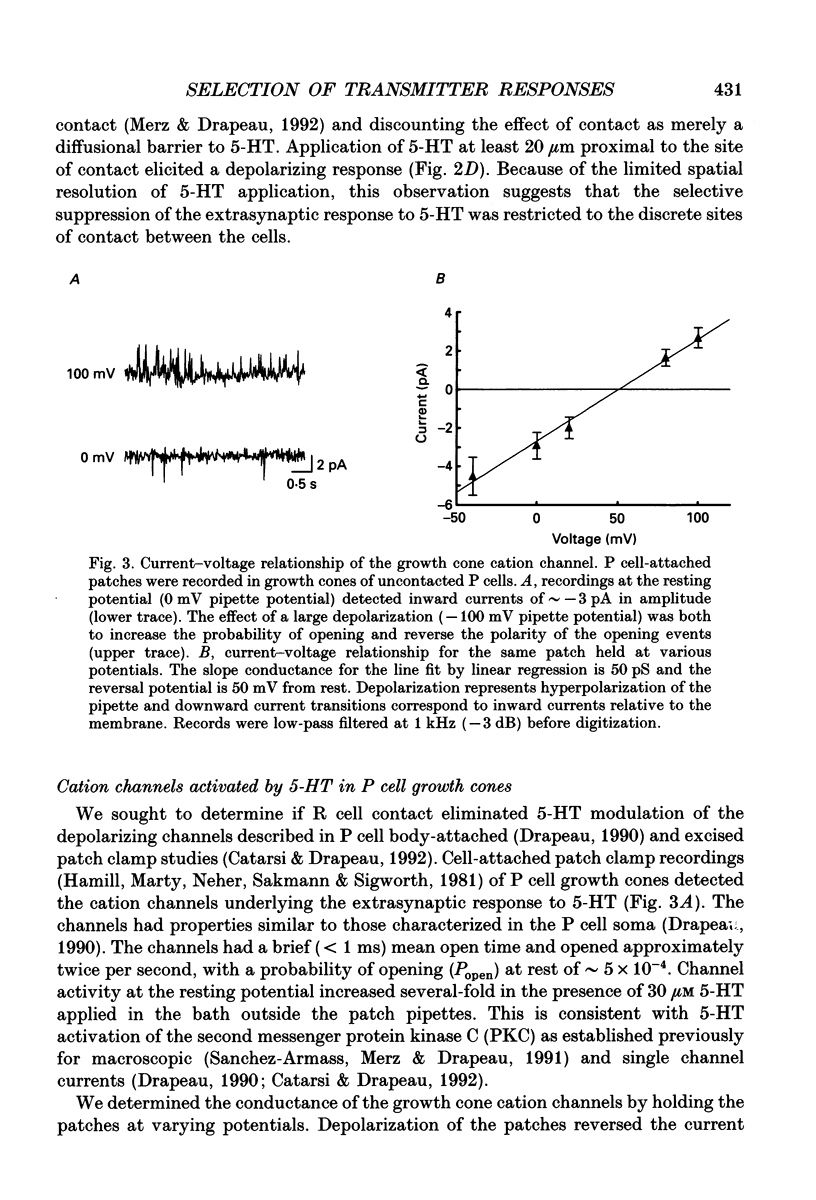
Abstract
1. Pressure sensitive (P) neurons of the leech Hirudo medicinalis show both an inhibitory, Cl(-)-dependent response and a depolarizing, cationic response to pipette application of serotonin (5-HT). Serotonergic Retzius (R) neurons in culture reform inhibitory, Cl(-)-dependent synapses with P neurons but fail to elicit the extrasynaptic, depolarizing response to 5-HT. We have examined the localization of the selection of 5-HT responses by testing the sensitivity of P cell growth cones and neurites to 5-HT application. 2. As measured by intracellular recording at the P cell soma, synaptic release of 5-HT from R cell processes activated only the Cl(-)-dependent response in P cell neurites. Focal application of 5-HT from a micropipette depolarized uncontacted P cell growth cones and neurites. In contrast, processes from the same P cells that were contacted by R cells were rarely depolarized by 5-HT application unless the application pipette was moved along the neurites away from the sites of contact. 3. The channels underlying the depolarizing response to 5-HT were identified in patch clamp recordings from P cell growth cones. These cation channels showed rare, brief openings in the absence of 5-HT. Application of 5-HT in the bath (outside the patch pipette) increased channel activity in uncontacted P cell growth cones but not in growth cones of the same P cells contacted by R cells. 4. We conclude that the selection of transmitter responses during synapse formation was localized to discrete sites of contact between the synaptic partners.
Full text
PDF



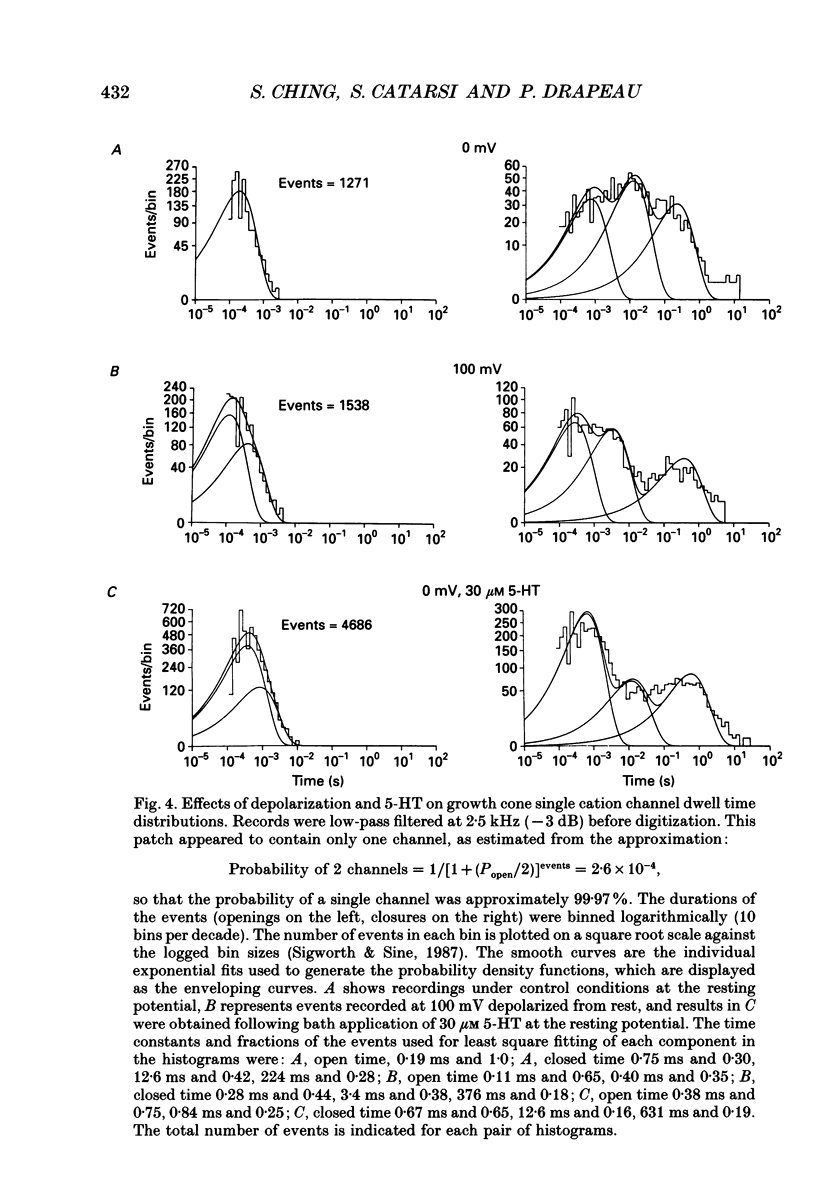
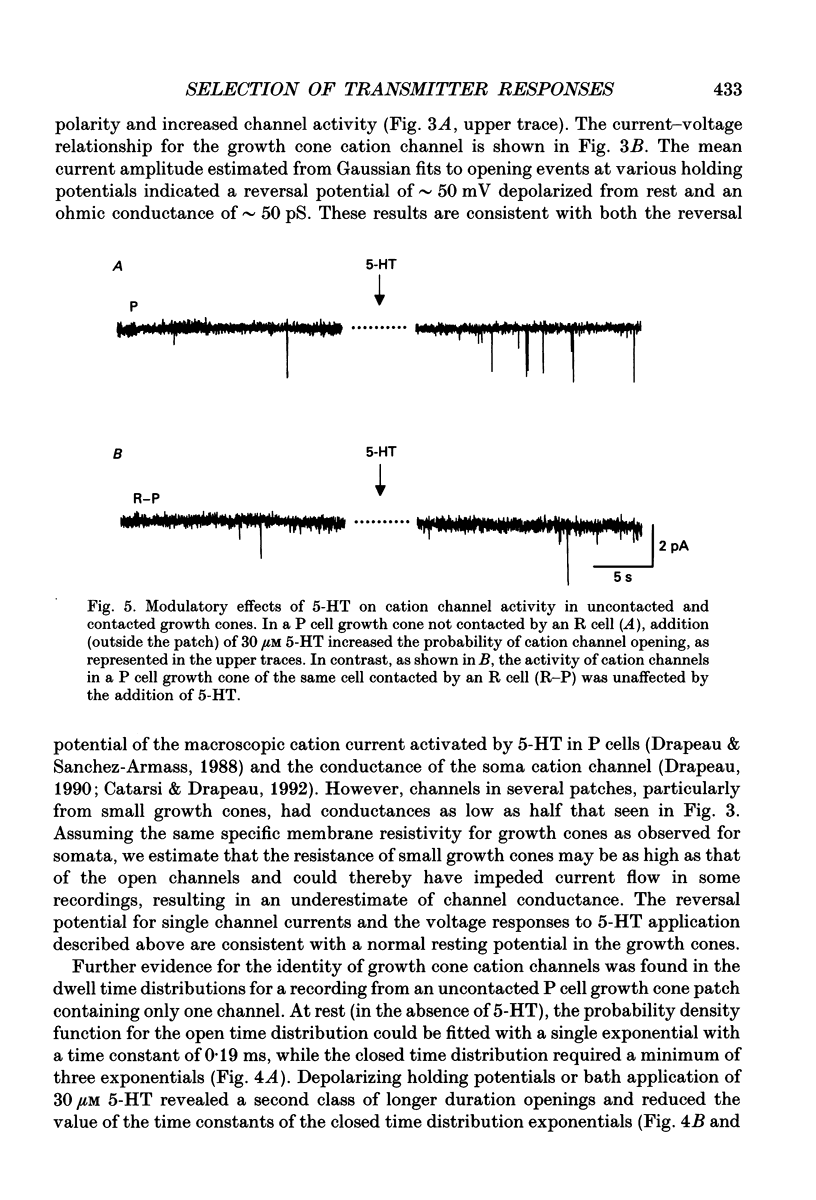






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Cohen M. W. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardetti F., Schacher S., Siegelbaum S. A. Action potentials, macroscopic and single channel currents recorded from growth cones of Aplysia neurones in culture. J Physiol. 1986 May;374:289–313. doi: 10.1113/jphysiol.1986.sp016080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. K., Boyd R. T., Halvorsen S. W., Higgins L. S., Jacob M. H., Margiotta J. F. Regulating the number and function of neuronal acetylcholine receptors. Trends Neurosci. 1989 Jan;12(1):16–21. doi: 10.1016/0166-2236(89)90151-3. [DOI] [PubMed] [Google Scholar]
- Bloch R. J., Pumplin D. W. Molecular events in synaptogenesis: nerve-muscle adhesion and postsynaptic differentiation. Am J Physiol. 1988 Mar;254(3 Pt 1):C345–C364. doi: 10.1152/ajpcell.1988.254.3.C345. [DOI] [PubMed] [Google Scholar]
- Catarsi S., Drapeau P. Loss of extrasynaptic channel modulation by protein kinase C underlies the selection of serotonin responses in an identified leech neuron. Neuron. 1992 Feb;8(2):275–281. doi: 10.1016/0896-6273(92)90294-n. [DOI] [PubMed] [Google Scholar]
- Chiquet M., Nicholls J. G. Neurite outgrowth and synapse formation by identified leech neurones in culture. J Exp Biol. 1987 Sep;132:191–206. doi: 10.1242/jeb.132.1.191. [DOI] [PubMed] [Google Scholar]
- Chow I. Cell-cell interaction during synaptogenesis. J Physiol (Paris) 1990;84(1):121–127. [PubMed] [Google Scholar]
- Dietzel I. D., Drapeau P., Nicholls J. G. Voltage dependence of 5-hydroxytryptamine release at a synapse between identified leech neurones in culture. J Physiol. 1986 Mar;372:191–205. doi: 10.1113/jphysiol.1986.sp016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau P. Loss of channel modulation by transmitter and protein kinase C during innervation of an identified leech neuron. Neuron. 1990 Jun;4(6):875–882. doi: 10.1016/0896-6273(90)90140-b. [DOI] [PubMed] [Google Scholar]
- Drapeau P., Melinyshyn E., Sanchez-Armass S. Contact-mediated loss of the nonsynaptic response to transmitter during reinnervation of an identified leech neuron in culture. J Neurosci. 1989 Jul;9(7):2502–2508. doi: 10.1523/JNEUROSCI.09-07-02502.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau P., Sanchez-Armass S. Parallel processing and selection of the responses to serotonin during reinnervation of an identified leech neuron. J Neurobiol. 1989 Jul;20(5):312–325. doi: 10.1002/neu.480200505. [DOI] [PubMed] [Google Scholar]
- Drapeau P., Sanchez-Armass S. Selection of postsynaptic serotonin receptors during reinnervation of an identified leech neuron in culture. J Neurosci. 1988 Dec;8(12):4718–4727. doi: 10.1523/JNEUROSCI.08-12-04718.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easter S. S., Jr, Purves D., Rakic P., Spitzer N. C. The changing view of neural specificity. Science. 1985 Nov 1;230(4725):507–511. doi: 10.1126/science.4048944. [DOI] [PubMed] [Google Scholar]
- Falls D. L., Harris D. A., Johnson F. A., Morgan M. M., Corfas G., Fischbach G. D. Mr 42,000 ARIA: a protein that may regulate the accumulation of acetylcholine receptors at developing chick neuromuscular junctions. Cold Spring Harb Symp Quant Biol. 1990;55:397–406. doi: 10.1101/sqb.1990.055.01.040. [DOI] [PubMed] [Google Scholar]
- Fontaine B., Klarsfeld A., Hökfelt T., Changeux J. P. Calcitonin gene-related peptide, a peptide present in spinal cord motoneurons, increases the number of acetylcholine receptors in primary cultures of chick embryo myotubes. Neurosci Lett. 1986 Oct 30;71(1):59–65. doi: 10.1016/0304-3940(86)90257-0. [DOI] [PubMed] [Google Scholar]
- Fuchs P. A., Henderson L. P., Nicholls J. G. Chemical transmission between individual Retzius and sensory neurones of the leech in culture. J Physiol. 1982 Feb;323:195–210. doi: 10.1113/jphysiol.1982.sp014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. Q., Macagno E. R. Extension and retraction of axonal projections by some developing neurons in the leech depends upon the existence of neighboring homologues. I. The HA cells. J Neurobiol. 1987 Jan;18(1):43–59. doi: 10.1002/neu.480180105. [DOI] [PubMed] [Google Scholar]
- Gao W. Q., Macagno E. R. Extension and retraction of axonal projections by some developing neurons in the leech depends upon the existence of neighboring homologues. II. The AP and AE neurons. J Neurobiol. 1987 May;18(3):295–313. doi: 10.1002/neu.480180305. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Paupardin-Tritsch D. On the transmitter function of 5-hydroxytryptamine at excitatory and inhibitory monosynaptic junctions. J Physiol. 1974 Dec;243(2):457–481. doi: 10.1113/jphysiol.1974.sp010762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I., Rubin G. M. Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992 Jan 24;68(2):271–281. doi: 10.1016/0092-8674(92)90470-w. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Haydon P. G., Cohan C. S., McCobb D. P., Miller H. R., Kater S. B. Neuron-specific growth cone properties as seen in identified neurons of Helisoma. J Neurosci Res. 1985;13(1-2):135–147. doi: 10.1002/jnr.490130110. [DOI] [PubMed] [Google Scholar]
- Haydon P. G., McCobb D. P., Kater S. B. Serotonin selectively inhibits growth cone motility and synaptogenesis of specific identified neurons. Science. 1984 Nov 2;226(4674):561–564. doi: 10.1126/science.6093252. [DOI] [PubMed] [Google Scholar]
- Haydon P. G., Zoran M. J. Formation and modulation of chemical connections: evoked acetylcholine release from growth cones and neurites of specific identified neurons. Neuron. 1989 May;2(5):1483–1490. doi: 10.1016/0896-6273(89)90194-3. [DOI] [PubMed] [Google Scholar]
- Henderson L. P. The role of 5-hydroxytryptamine as a transmitter between identified leech neurones in culture. J Physiol. 1983 Jun;339:309–324. doi: 10.1113/jphysiol.1983.sp014718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990 Nov;5(5):555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Role L. W., Fischbach G. D. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983 Oct 13;305(5935):632–634. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killisch I., Dotti C. G., Laurie D. J., Lüddens H., Seeburg P. H. Expression patterns of GABAA receptor subtypes in developing hippocampal neurons. Neuron. 1991 Dec;7(6):927–936. doi: 10.1016/0896-6273(91)90338-z. [DOI] [PubMed] [Google Scholar]
- Kristan W. B., Jr, Nusbaum M. P. The dual role of serotonin in leech swimming. J Physiol (Paris) 1982;78(8):743–747. [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. Y., Tsien R. W. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Nicholls J. Steps in the development of chemical and electrical synapses by pairs of identified leech neurons in culture. Proc R Soc Lond B Biol Sci. 1989 Apr 22;236(1284):253–268. doi: 10.1098/rspb.1989.0023. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Drapeau P. Cell-specific contact selects transmitter responses in an identified leech neuron. Proc Biol Sci. 1992 May 22;248(1322):129–133. doi: 10.1098/rspb.1992.0052. [DOI] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New H. V., Mudge A. W. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. 1986 Oct 30-Nov 5Nature. 323(6091):809–811. doi: 10.1038/323809a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Nitkin R. M., Smith M. A., Magill C., Fallon J. R., Yao Y. M., Wallace B. G., McMahan U. J. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J Cell Biol. 1987 Dec;105(6 Pt 1):2471–2478. doi: 10.1083/jcb.105.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Lague P. H., Huttner S. L., Vandenberg C. A., Morrison-Graham K., Horn R. Morphological properties and membrane channels of the growth cones induced in PC12 cells by nerve growth factor. J Neurosci Res. 1985;13(1-2):301–321. doi: 10.1002/jnr.490130120. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Pellegrino M., Simonneau M. Distribution of receptors for acetylcholine and 5-hydroxytryptamine on identified leech neurones growing in culture. J Physiol. 1984 Jul;352:669–684. doi: 10.1113/jphysiol.1984.sp015316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroutka S. J. 5-Hydroxytryptamine receptor subtypes. Annu Rev Neurosci. 1988;11:45–60. doi: 10.1146/annurev.ne.11.030188.000401. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Aréchiga H., Nicholls J. G. Influence of substrate on the distribution of calcium channels in identified leech neurons in culture. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4075–4078. doi: 10.1073/pnas.85.11.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Armass S., Merz D. C., Drapeau P. Distinct receptors, second messengers and conductances underlying the dual responses to serotonin in an identified leech neurone. J Exp Biol. 1991 Jan;155:531–547. doi: 10.1242/jeb.155.1.531. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Wisden W., Verdoorn T. A., Pritchett D. B., Werner P., Herb A., Lüddens H., Sprengel R., Sakmann B. The GABAA receptor family: molecular and functional diversity. Cold Spring Harb Symp Quant Biol. 1990;55:29–40. doi: 10.1101/sqb.1990.055.01.006. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. Impulse activity and the patterning of connections during CNS development. Neuron. 1990 Dec;5(6):745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit J., Lux H. D. Distribution of calcium currents in sprouting PC12 cells. J Neurosci. 1989 Dec;9(12):4190–4199. doi: 10.1523/JNEUROSCI.09-12-04190.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A., Cluzeaud F., Pfeiffer F., Betz H., Korn H. Distribution of glycine receptors at central synapses: an immunoelectron microscopy study. J Cell Biol. 1985 Aug;101(2):683–688. doi: 10.1083/jcb.101.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. G., Qu Z., Huganir R. L. Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron. 1991 Jun;6(6):869–878. doi: 10.1016/0896-6273(91)90227-q. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Brenner H. R., Sakmann B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular synapses. J Cell Biol. 1991 Jul;114(1):125–141. doi: 10.1083/jcb.114.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. H., Poo M. M. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature. 1983 Oct 13;305(5935):634–637. doi: 10.1038/305634a0. [DOI] [PubMed] [Google Scholar]