Abstract
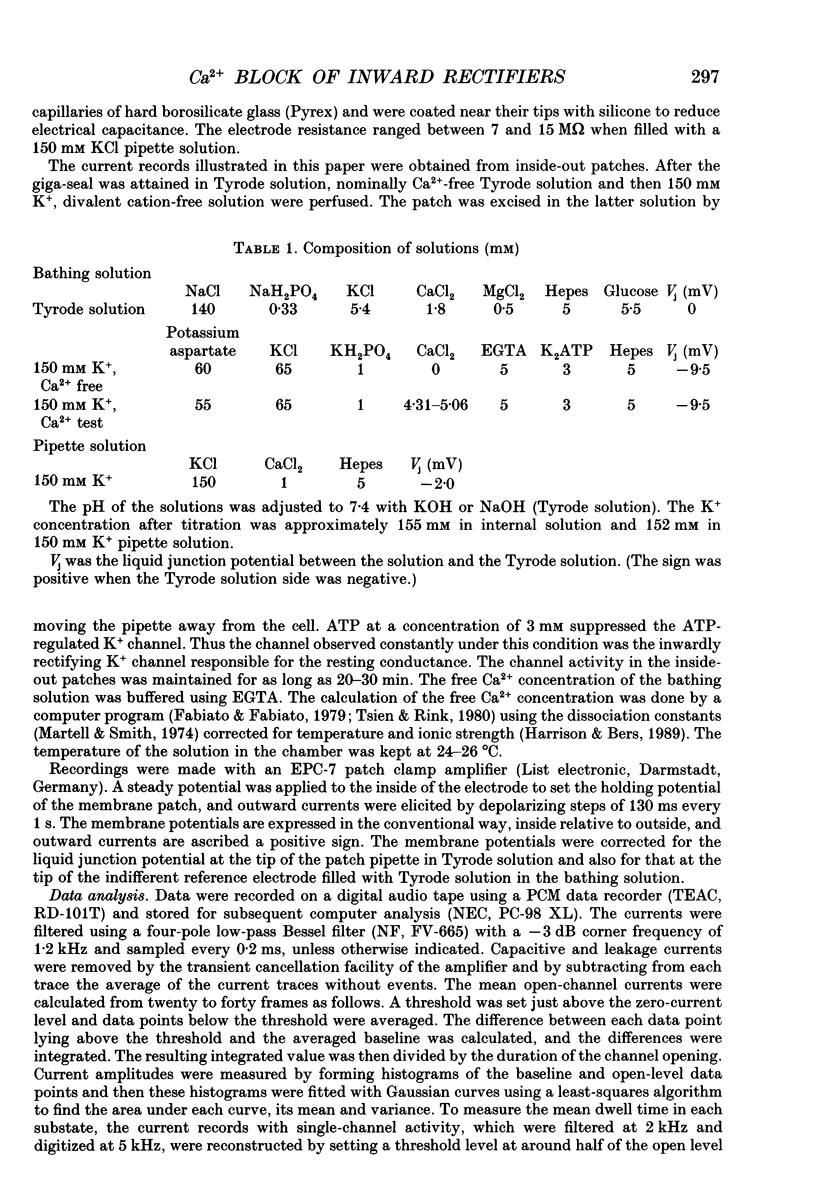
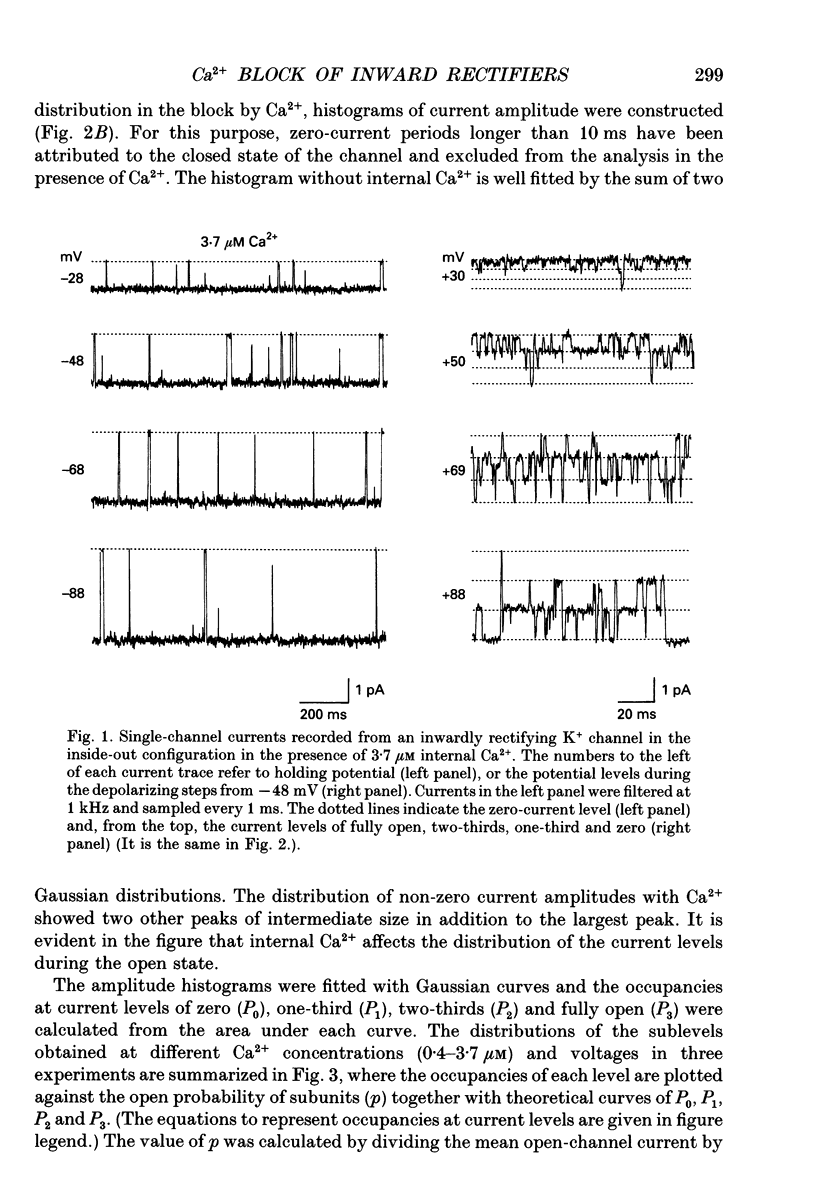
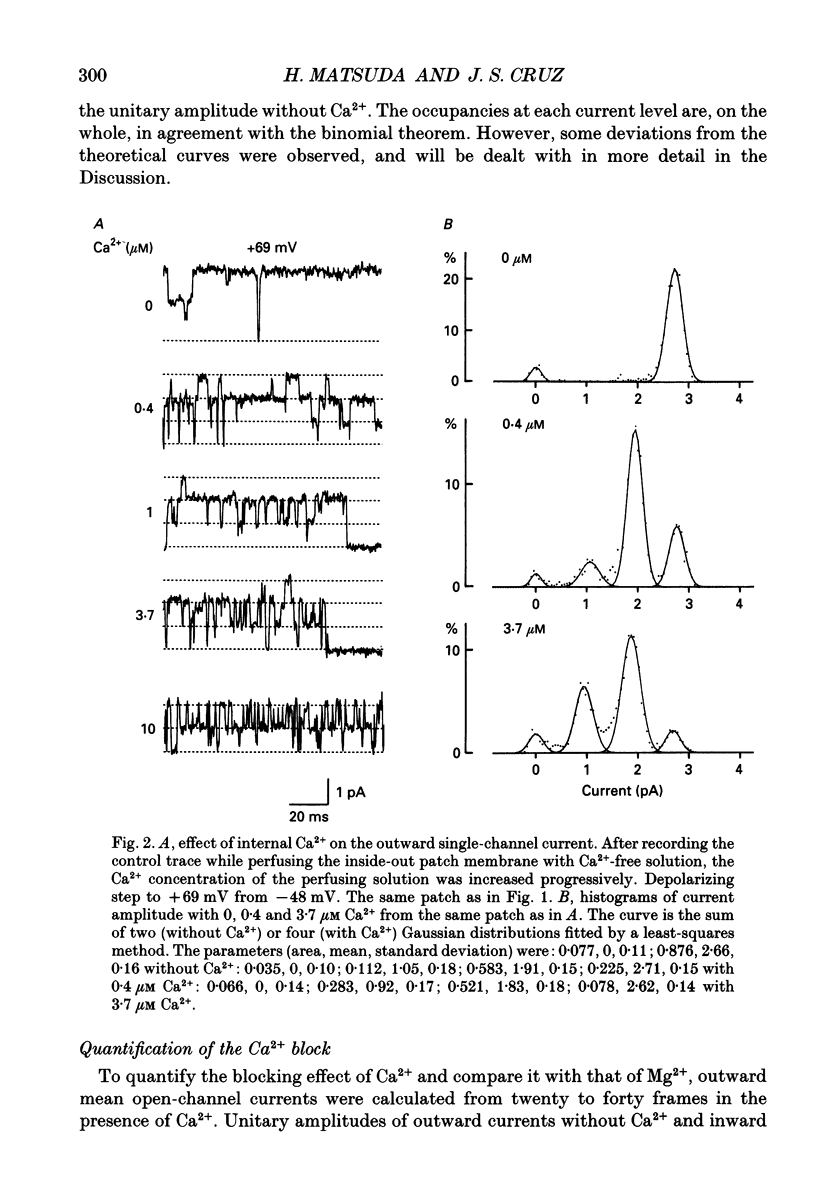
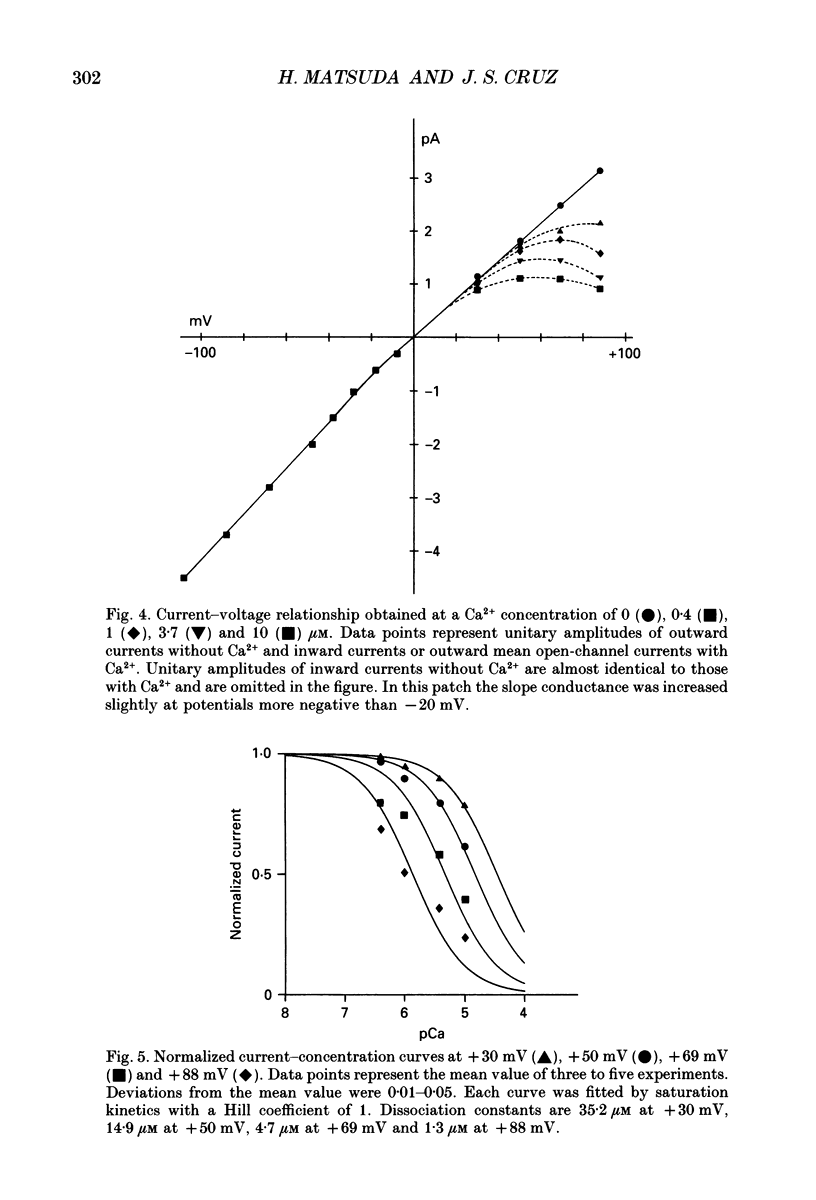
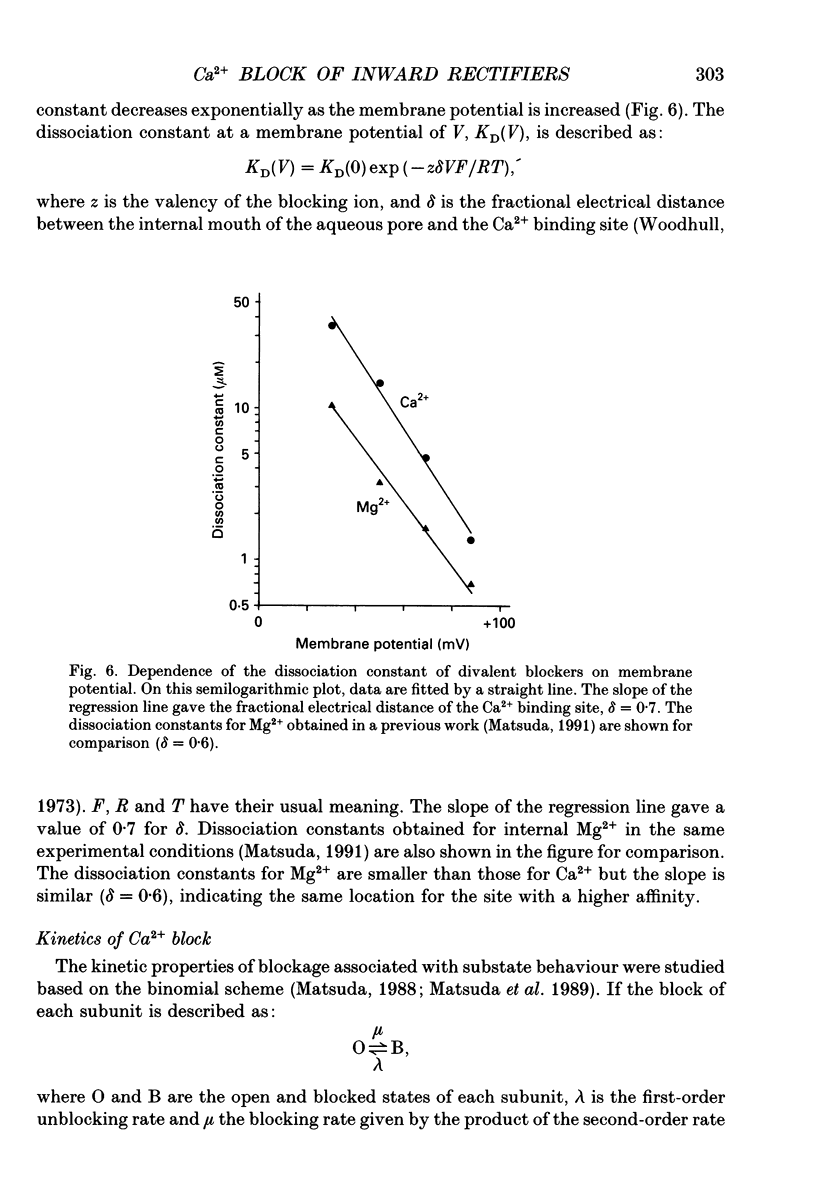
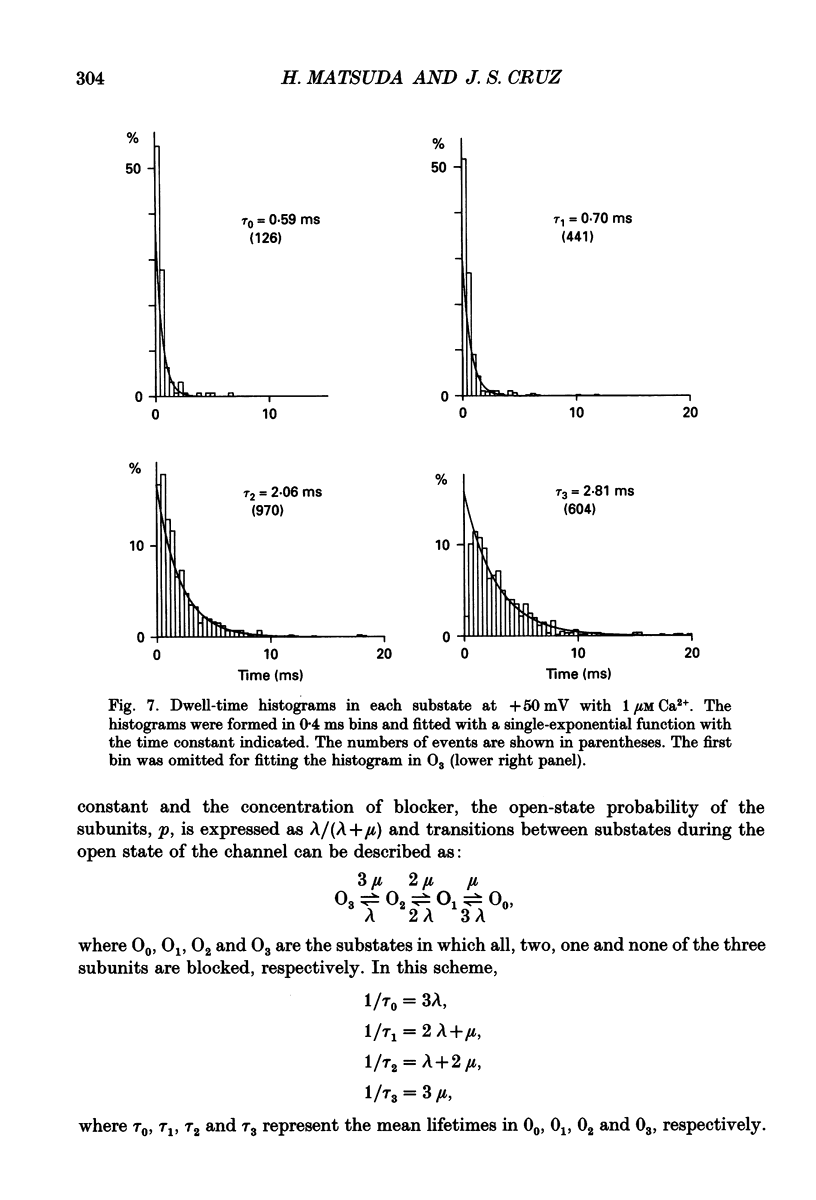
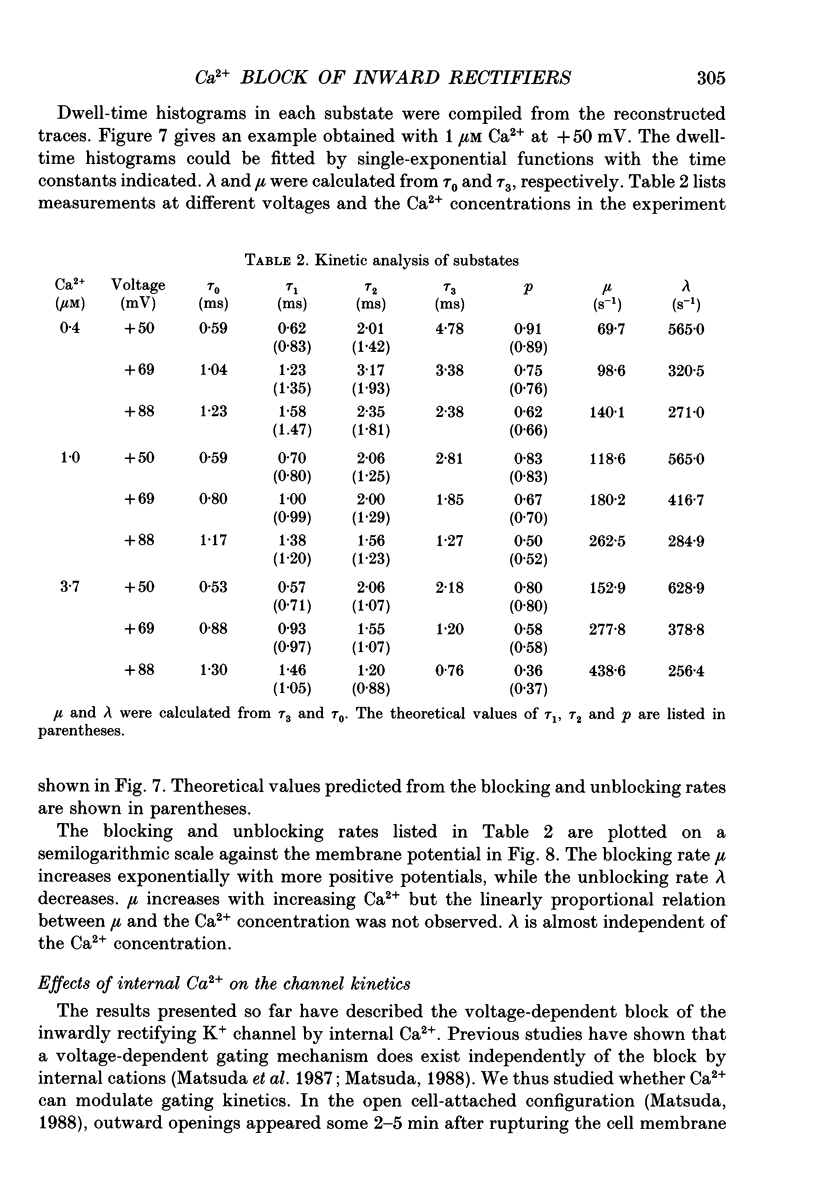
1. The block of the inwardly rectifying K+ channel by intracellular Ca2+ was studied in guinea-pig ventricular cells. 2. Single-channel currents through the inwardly rectifying K+ channel were recorded in the inside-out configuration at 150 mM external and internal K+. Internal Ca2+, at a concentration of 0.4-10 microM, induced subconductance levels with one-third and two-thirds of the unitary amplitude in the outward currents without affecting the inward currents. 3. Occupancy at each sublevel was estimated from the amplitude histogram which showed four equally spaced peaks in the presence of internal Ca2+. At different degrees of blockade, the distribution of the current levels showed a reasonable agreement with the binomial theorem. 4. The outward mean open-channel currents were measured at different Ca2+ concentrations and voltages. The current-voltage relation rectified inwardly in the presence of internal Ca2+ in a concentration-dependent manner. 5. The outward mean open-channel currents were normalized to unitary amplitudes in the absence of Ca2+. The normalized current-Ca2+ concentration curve was fitted by saturation kinetics with a Hill coefficient of 1 at each voltage. The voltage dependence of the dissociation constants gives the value for the fractional electrical distance of the Ca2+ binding site of 0.7. 6. The dwell times in each substrate were distributed exponentially. On the assumption that the inwardly rectifying K+ channel of cardiac cells is composed of three identical conducting subunits and each subunit is blocked by Ca2+ independently, the blocking (mu) and unblocking (lambda) rates were calculated. The value of mu increased with higher Ca2+ concentrations or larger depolarizations, while lambda was independent of Ca2+ and decreased with larger depolarization. 7. It is thus concluded that internal Ca2+ produces a voltage-dependent block of the channel to cause inward rectification although the blocking effect is less potent than that of Mg2+. The substate behaviour seen with internal Ca2+ supports the triple-barrelled structure of the cardiac inwardly rectifying K+ channel.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatter L. A., McGuigan J. A. Intracellular pH regulation in ferret ventricular muscle. The role of Na-H exchange and the influence of metabolic substrates. Circ Res. 1991 Jan;68(1):150–161. doi: 10.1161/01.res.68.1.150. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Findlay I. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 1987 Oct;410(3):313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison S. M., Bers D. M. Correction of proton and Ca association constants of EGTA for temperature and ionic strength. Am J Physiol. 1989 Jun;256(6 Pt 1):C1250–C1256. doi: 10.1152/ajpcell.1989.256.6.C1250. [DOI] [PubMed] [Google Scholar]
- Hongo K., Tanaka E., Kurihara S. Alterations in contractile properties and Ca2+ transients by beta-and muscarinic receptor stimulation in ferret myocardium. J Physiol. 1993 Feb;461:167–184. doi: 10.1113/jphysiol.1993.sp019507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H. Dual effects of intracellular magnesium on muscarinic potassium channel current in single guinea-pig atrial cells. J Physiol. 1989 Jan;408:313–332. doi: 10.1113/jphysiol.1989.sp017461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei M., Noma A., Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Effects of external and internal K+ ions on magnesium block of inwardly rectifying K+ channels in guinea-pig heart cells. J Physiol. 1991 Apr;435:83–99. doi: 10.1113/jphysiol.1991.sp018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Effects of internal and external Na+ ions on inwardly rectifying K+ channels in guinea-pig ventricular cells. J Physiol. 1993 Jan;460:311–326. doi: 10.1113/jphysiol.1993.sp019473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Matsuura H., Noma A. Triple-barrel structure of inwardly rectifying K+ channels revealed by Cs+ and Rb+ block in guinea-pig heart cells. J Physiol. 1989 Jun;413:139–157. doi: 10.1113/jphysiol.1989.sp017646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Open-state substructure of inwardly rectifying potassium channels revealed by magnesium block in guinea-pig heart cells. J Physiol. 1988 Mar;397:237–258. doi: 10.1113/jphysiol.1988.sp016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Ca modulates outward current through IK1 channels. J Membr Biol. 1990 Jun;116(1):41–45. doi: 10.1007/BF01871670. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DiFrancesco D. Intracellular Ca modulates K-inward rectification in cardiac myocytes. Pflugers Arch. 1989 Jan;413(3):322–324. doi: 10.1007/BF00583549. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Kimitsuki T., Noma A. Conductance properties of the Na(+)-activated K+ channel in guinea-pig ventricular cells. J Physiol. 1991 Feb;433:241–257. doi: 10.1113/jphysiol.1991.sp018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Isenberg G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J Physiol. 1991 Apr;435:349–372. doi: 10.1113/jphysiol.1991.sp018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


