Abstract
Purpose
This paper provides an overview of the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) cohort and biobank, including baseline characteristics of participants enrolled up to 2023, and post-enrollment rates of cardiovascular disease outcomes and mortality.
Methods
Since 2010, the DD2 project has enrolled individuals with type 2 diabetes mellitus (T2DM) recently diagnosed by general practitioners and by hospital-based clinicians across Denmark. Data from questionnaires, clinical examinations, and biological samples are collected at enrollment. Additional baseline and longitudinal follow-up data are accessed via linkage to health registries.
Results
Between 2010 and 2023, the DD2 project enrolled 11,369 participants (41.3% women, median age 61.4 years). Median T2DM duration at enrollment was 1.3 years, and median BMI was 31.6 kg/m2 for women and 30.5 kg/m2 for men. 18.3% were smokers, 5.7% consumed more than 14/21 units of alcohol weekly (women/men), and 17.9% reported leisure-time physical inactivity. Original midwife records dating back >80 years revealed that 20.2% of cohort participants had birth weights <3000 g. Based on complete hospital contact history 10 years before enrollment, 20.7% of cohort participants had macrovascular complications, 17.0% had microvascular complications, and 21.7% had kidney disease based on eGFR or urine albumin-creatinine measurements. At enrollment, statins were used by 68.2%, antihypertensive drugs by 69.9%, and glucose-lowering drugs by 86.5% of individuals. Median HbA1c was 48 mmol/mol and median LDL cholesterol 2.2 mmol/L. Genome-wide genotyping and biomarker data have been analyzed for over 9000 individuals. During the current follow-up time from the enrollment date (median 7.9 years), incident cardiovascular disease rate has been 13.8 per 1000 person-years and the mortality rate has been 17.6 per 1000 person-years.
Conclusion
The DD2 cohort, with its detailed information and long-term follow up, can improve our understanding of the progression and prevention of complications among individuals with newly diagnosed T2DM.
Keywords: cohort profile, type 2 diabetes mellitus, patient characteristics, Denmark
Introduction
The population prevalence of type 2 diabetes mellitus (T2DM) has more than doubled in many countries over the last two decades,1,2 driven mainly by unhealthy lifestyle behaviors and increasing obesity rates.1 In Denmark, 320,000 people may now have T2DM,3 and the attributable healthcare costs are high.4 The costs are mainly driven by T2DM complications including cardiovascular disease (CVD), cancer, and end-stage kidney disease.4,5 To prevent T2DM complications, we need an in-depth understanding of the heterogeneity in the etiology, clinical presentation, and prognosis of T2DM.6 Clinical cohorts and biobanks nested in comprehensive healthcare systems with complete long-term follow-up such as in the Nordic countries can improve our understanding of the determinants of complications and life course trajectories in persons with newly diagnosed T2DM.
The Danish Centre for Strategic Research in Type 2 Diabetes Project (DD2), established in 2010, was designed as an international resource for research into T2DM.7 The project’s primary objective is to study the clinical course of people with newly diagnosed T2DM, including progression, complications, identification of clinical meaningful subgroups, and factors predicting patient prognosis.7 The DD2 Project provides a high-quality research cohort that allows for detailed clinical characterization of individuals with newly diagnosed T2DM.8–11 It also supports the development of more personalized treatments and facilitates examination of therapeutic interventions in routine clinical care to impede development and progression of diabetes complications.
Denmark has a long-standing tradition of recording individual-level data in clinical, health, and administrative registries, which have been utilized in a broad spectrum of epidemiological studies.12 The DD2 cohort includes interview and clinical examination data from cohort participants, complemented by a biobank containing blood and urine samples. These data are continually linked to a comprehensive network of the Danish nationwide routine social and health registries. The most recent description of the DD2 cohort, published in 2018, presented data on the first 7011 participants enrolled between 2010 and 2016.13 Since then, the DD2 cohort has expanded to include data on more than 11,000 participants. Several additional data sources have been linked successfully with the cohort, and nested research studies have provided results for analyses of several selected biomarkers and genome-wide genotype data.
The aims of the present Cohort Profile paper are twofold. First, in the Material and Method sections, we describe the DD2 cohort enrollment procedures, current data linkage systems, and the numerous old and new (since 2018) data sources that are linked and available in the DD2 Project. Second, in the Results section, we provide an up-to-date description of baseline characteristics for all participants enrolled in the DD2 cohort from 2010 through 2023, and then provide post-enrollment follow-up rates for CVD outcomes and all-cause mortality.
Material and Methods
Description of DD2 Enrollment, Data Linkage, and Old and New Data Sources
Study Participants, Enrollment Procedure, and Ethics
Since 2010, the DD2 project has enrolled individuals with T2DM recently diagnosed by general practitioners or hospital outpatient clinics in Denmark. Eligible participants are aged 18 years or older and have received a T2DM diagnosis in routine clinical care within the previous two years (between 2012 and 2016, participants with a diabetes duration of more than two years were also eligible). The enrollment procedure, previously described in detail,13–16 includes the participant signing a written informed consent document, completing a brief online questionnaire, and undergoing a short clinical examination. Participants retain the right to withdraw from the DD2 cohort at any time. The DD2 project has received approval from the Danish Regional Ethics Committee on Health Research for Southern Denmark (record no. S-20100082) and the Danish Data Protection Agency (record nos. 2008–58-0035 and 2016–051-000001/2514).
DD2 Data and Data Linkage
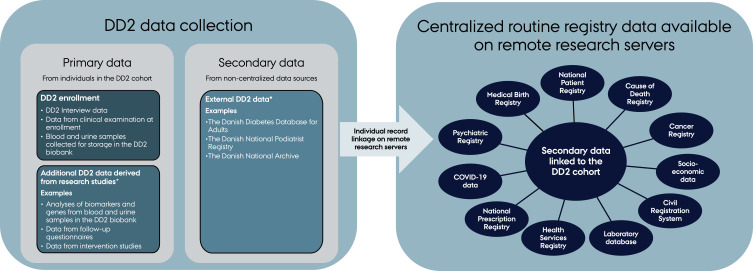
The unique civil registration number assigned by the Danish Civil Registration System to all Danish residents at birth or upon immigration allows individual-level linkage among registries in Denmark.17 Figure 1 presents an overview of the data structure and data sources available in the linked DD2 cohort. The centralized registry data are available on remote research servers maintained by the two main health data organizations in Denmark: Statistics Denmark and the Danish Health Data Authority. DD2 data are regularly uploaded to these research servers and linked to allow access to a complete hospital contact history, routine care laboratory test results, prescription drug use, socioeconomic markers, and lifelong follow up for health outcomes after DD2 enrollment. The following sections provide a more detailed overview of the different data sources.
Figure 1.
Overview of data sources in the DD2 cohort.
Notes: *These data can be accessed through collaboration with DD2 research groups.
Abbreviation: COVID-19, Coronavirus Disease-2019.
Primary DD2 Data Collected at Enrollment
Participants complete a brief online questionnaire that includes information on their leisure-time physical activity level, and alcohol consumption (above or below 14/21 units of alcohol per week, which was the recommended weekly limit for women and men in 2010 when DD2 was established), height (added in 2015), weight at 20 years of age, maximum lifetime weight reached before enrollment, and family history of diabetes. During the physical examination, the clinician records weight (added in 2015), hip and waist circumference, resting heart rate, and a clinical assessment of the month and year of first-time T2DM diagnosis (added in 2013). Each participant is asked to provide a blood sample after an overnight fast and a morning urine sample on the day of the examination or as soon as possible. The procedure for collecting and storing the biological samples has been described previously.14,18 All blood and urine samples are stored in the DD2 biobank, and analysis of samples generally depends on funding provided through subsequent research studies.
Additional DD2 Data Derived from Research Studies
Research studies nested within the DD2 cohort have the potential to generate new primary data. Several studies have analyzed existing biological samples.9,11,13,19–21 The completeness and availability of these data depend on the time points at which research studies generated the results. For participants enrolled in the DD2 cohort between 2010 and 2021, plasma and serum samples have been analyzed for a range of biomarkers, and genome-wide chip genotyping has been performed on their blood samples. A description of the genotyping procedure is provided in the Supplementary Materials. Other studies have collected primary follow-up data in the cohort, eg new data on lifestyle and body composition, data on neuropathy and mental health etc. (examples are provided in the Supplementary Materials).13,22–26
Secondary DD2 Data Accessed from Non-Centralized Data Sources
Since the inception of the DD2 project in 2010, linkage to external data sources has been considered a crucial source of data that are resource-intensive to collect in clinical practice and unavailable in the Danish health registries. For instance, linkage to the Danish Diabetes Database of Adults (DDDA) provides additional and repeated data on smoking status, blood pressure, and HbA1c, which are key variables not obtained at DD2 enrollment.27 The DDDA, now replaced with the Danish Diabetes Database, is a quality-of-care database that collects detailed information on patients with diabetes from general practitioners and diabetes outpatient hospital clinics in Denmark.27 However, the DDDA is incomplete because not all healthcare providers supply data to this database.27 This has affected the availability of records for DD2 participants in the DDDA, as only 80% of the DD2 cohort has registered DDDA data. Moreover, the DDDA monitors quality of care only once per year, so the timing of its data collection may not coincide with the DD2 enrollment date.
The DD2 cohort is also linked to the Podiatrist Database. This database, established in 2011, records information on examinations performed by Danish podiatrists working under a collective agreement with the Danish Administrative Regions. Records include symptoms of neuropathy and information from a clinical examination of the lower extremities, including circulatory and neuropathy status. The Podiatrist Database provides data on DD2 participants enrolled between 2011 and 2022. Approximately 60% of DD2 participants have a record in the database. DD2 participants without records may have had foot examinations by other healthcare professionals (eg, diabetes outpatient clinics, general practitioners), a private podiatrist not working under the collective agreement or may not have been examined at all.
As part of the “Closing in on Sub-segmentation in Type 2 diabetes in the Danish Nationwide DD2 Cohort project (DD2 sub-segmentation project)”,11 the DD2 cohort is linked to birth-related data from midwife paper records maintained by the Danish National Archive. The Danish National Archive provided birth-related data on DD2 participants born in Denmark between 1920 and 1988. The data consist of birthweight, birth length, birth at term (yes/no, and if no, the number of weeks born before term), and twin status (yes/no).
Routine Registry Data Available on Remote Research Servers
The DD2 cohort is linked on an individual level to a variety of Danish health and administrative registries (Figure 1). The Danish Civil Registration System has recorded vital and migration status daily for all Danish residents since April 1968.17 The Danish National Patient Registry has recorded information on all Danish inpatient hospitalizations since 1977, and on all outpatient clinic and emergency department visits since 1995.28 The Danish National Prescription Registry has recorded detailed data on prescriptions dispensed from all Danish community pharmacies since 1995.29 The Register of Laboratory Results for Research has recorded laboratory results from hospital laboratory information systems, which have covered all hospital departments and general practitioners in Denmark since 2015.30 In addition to these registries, the DD2 cohort is linked to the Danish Cancer Registry,31 the Psychiatric Central Research Registry,32,33 socioeconomic registries at Statistics Denmark (ie, the Educational Attainment Registry,34 Integrated Database for Labor Market Research,35 Income Statistics Register,36 Household and Family Register,17 and the Register of Social Benefits and other Transfer Payments37), the National Health Insurance Service Registry,38 the Medical Birth Registry,39 COVID-19 test results and vaccinations,40 and the Cause of Death Registry.41 Linkage to other registries and databases is possible with approval of data permission applications sent to Statistics Denmark or the Danish Health Data Authority.
Updated Analyses of the Present DD2 Cohort, Including Baseline Characteristics at Enrollment and Longitudinal Follow-Up Data
Study Cohort
For this cohort profile paper, we included all participants in the DD2 cohort as of April 13, 2023. We linked the DD2 data with the data sources shown in Figure 1, with a special focus on data from DDDA, the Danish Civil Registration System, the Danish National Patient Registry, the Danish National Prescription Registry, the Register of Laboratory Results for Research, and the Educational Attainment Registry.
Baseline Characteristics at Enrollment
We characterized participants according to demographic factors, body composition, lifestyle factors, comorbidities, diabetes and cardiovascular drug treatment, selected routine care laboratory results, and DD2 biomarkers. Supplementary Tables 1–6 present an overview of covariate definitions and look-back periods used in this study. For each covariate, we report the proportion of missing data at enrollment.
Follow-Up Analyses
We provide follow-up rates for selected clinical outcomes, focusing on all-cause mortality and a composite endpoint of CVD outcomes, including acute myocardial infarction, unstable angina, heart failure, coronary artery bypass graft/percutaneous coronary intervention procedures, stroke, and cerebral thrombolysis/thrombectomy procedures. Participants with a CVD hospital diagnosis recorded before enrollment were excluded from the CVD analysis. We followed participants from DD2 cohort enrollment until a CVD outcome, all-cause death, emigration, or the study’s end on May 31, 2023, whichever occurred first. We assessed mortality and CVD incidence rates per 1000 person-years and present cumulative incidences during follow-up.
Results
Characteristics at Enrollment: Demographics, Body Composition, and Lifestyle Factors
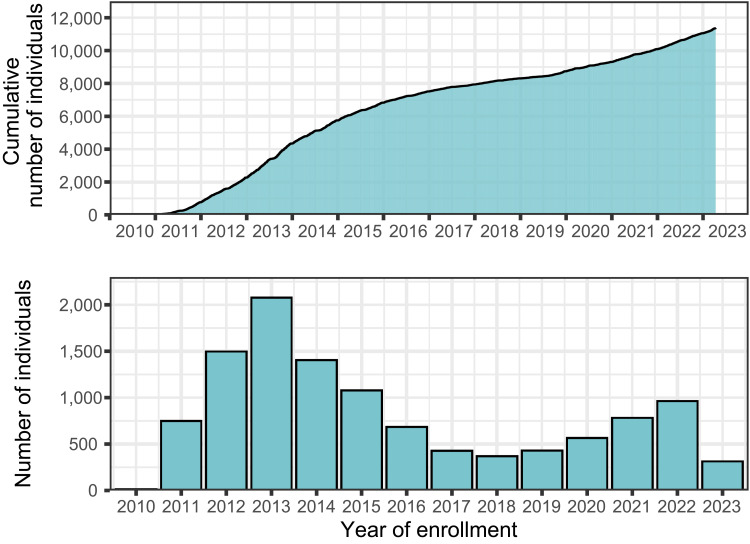
Between 2010 and 2023, the DD2 cohort enrolled 11,369 individuals with recently diagnosed T2DM, among whom 41.3% were women (Figure 2 and Table 1). Median age at enrollment was 61.4 years (quartiles 52.6–68.5 years) and median T2DM duration at time of enrollment was 1.3 years (quartiles 0.4–2.9 years). Educational attainment data were available for 10,020 individuals (88% of the cohort). Of these, 31.0% had a low, 50.0% had a moderate, and 19.1% had a high level of education, as defined by the International Standard Classification of Education. Data from midwife records were available for 8331 individuals (73% of the cohort). Of these, 20.2% had a birthweight of less than 3000g, 56.1% had a birthweight between 3000 and 3700 g, and 23.7% had a birthweight of more than 3700 g (Table 1).11
Figure 2.
Cumulative and annual number of participants enrolled in the DD2 project from 2010 to April 2023.
Table 1.
Distribution of Demographics, Body Composition, Lifestyle Behaviors, and Selected Additional Factors for Participants in the DD2 Cohort at Baseline Enrollment, Denmark, 2011–2023
| Characteristics | DD2 Cohort | Number with Non-Missing Data |
|---|---|---|
| Number of individuals | 11,369 (100.0%) | 11,369 (100.0%) |
| Women | 4697 (41.3%) | |
| Age, years | 61.4 (52.6–68.5) | 11,369 (100.0%) |
| Year of enrollment | 11,369 (100.0%) | |
| 2010–2013 | 4343 (38.2%) | |
| 2014–2016 | 3169 (27.9%) | |
| 2017–2019 | 1229 (10.8%) | |
| 2020–2023 | 2628 (23.1%) | |
| Region of residence at enrollment | 11,369 (100.0%) | |
| Capital Region | 1814 (16.0%) | |
| Central Denmark Region | 2506 (22.0%) | |
| North Denmark Region | 1160 (10.2%) | |
| Zealand Region | 1495 (13.1%) | |
| South Denmark Region | 4394 (38.6%) | |
| Civil status | 11,076 (97.4%) | |
| Single | 1614 (14.6%) | |
| Married/registered partnership | 6783 (61.2%) | |
| Divorced | 1822 (16.4%) | |
| Widow/widower | 857 (7.7%) | |
| Highest educational level | 10,020 (88%) | |
| Primary school/lower secondary school | 3102 (31.0%) | |
| Upper secondary school/short cycle tertiary school | 5002 (50.0%) | |
| Bachelor’s/Master’s/Doctoral degrees | 1916 (19.1%) | |
| Diabetes duration, years | 1.3 (0.4–2.9) | 11,369 (100.0%) |
| Presence of T2DM among immediate family | 6005 (52.8%) | 11,369 (100%) |
| Body composition (women = 4697 [100%]) | ||
| BMI, kg/m2 | 31.6 (27.3–36.4) | 3200 (68.1%) |
| BMI ≥35 kg/m2 | 996 (31.1%) | 3200 (68.1%) |
| Weight, kg | 85.0 (73.0–100.0) | 3526 (75.1%) |
| Weight at 20 years, kg | 60.0 (54.0–70.0) | 3912 (83.3%) |
| Maximum lifetime weight, kg | 93.0 (80.0–110.0) | 4553 (96.9%) |
| Hip circumference, cm | 111.0 (102.0–122.0) | 4553 (96.9%) |
| Waist circumference, cm | 104.0 (94.0–114.0) | 4556 (97.0%) |
| Body composition (men = 6672 [100%]) | ||
| BMI, kg/m2 | 30.5 (27.4–34.5) | 4603 (69.0%) |
| BMI ≥35 kg/m2 | 1051 (22.8%) | 4603 (69.0%) |
| Weight | 97.0 (86.0–111.0) | 5061 (75.9%) |
| Weight at 20 years, kg | 76.0 (70.0–85.0) | 5560 (83.3%) |
| Maximum lifetime weight, kg | 106.0 (94.0–120.0) | 6514 (97.6%) |
| Hip circumference, cm | 107.0 (101.0–114.0) | 6441 (96.5%) |
| Waist circumference, cm | 109.0 (100.0–119.0) | 6445 (96.6%) |
| Resting heart rate, beats/min | 70.0 (64.0–79.0) | 11,033 (97.0%) |
| Days per week with >30 min of physical activity | 11,319 (99.6%) | |
| 0–3 | 5561 (49.1%) | |
| 4–7 | 5758 (50.9%) | |
| Engaged in leisure-time sport activities | 4440 (39.3%) | 11,311 (99.5%) |
| Level of leisure-time physical activity during the past yeara | 11,319 (99.6%) | |
| Sedentary (eg, reading, watching television) | 2031 (17.9%) | |
| Some physical activity at least 4 h/week (eg, walking, cycling, or other light exercise) | 7082 (62.6%) | |
| Moderate physical activity at least 4 h/week (eg, heavy gardening, running, or swimming) | 2119 (18.7%) | |
| Vigorous physical activity several times/week (eg, running or soccer) | 87 (0.8%) | |
| Alcohol consumption >14 units (women) | 94 (2.0%) | 4660 (99.2%) |
| Alcohol consumption >21 units (men) | 550 (8.3%) | 6636 (99.5%) |
| Number of persons with available DDDA data | 9039 (79.5%) | 11,369 (100%) |
| Smoking status | 8137 (71.6%) | |
| Never smoker | 3722 (45.7%) | |
| Former smoker | 2928 (36.0%) | |
| Current smoker, occasionally | 87 (1.1%) | |
| Current smoker, daily | 1400 (17.2%) | |
| Systolic blood pressure, mmHg | 130.0 (124.0–140.0) | 6081 (53.5%) |
| Diastolic blood pressure, mmHg | 80.0 (75.0–86.0) | 6081 (53.5%) |
| Number of persons with available birthweight data | 9531 (83.8%) | 11,369 (100%) |
| Birthweight, grams | 8331 (73.3%) | |
| <3000 | 1685 (20.2%) | |
| 3000–3700 | 4675 (56.1%) | |
| >3700 | 1971 (23.7%) | |
| Number of patients with available podiatry data | 6732 (59.2%) | 11,369 (100%) |
| Contact with podiatrist at DD2 enrollment | 1984 (29.5%) | 6732 (59.2%) |
Notes: Continuous data are presented as medians and quartiles. Count data are presented as n and proportions (%). aSaltin–Grimby Physical Activity Level Scale. All covariate definitions are presented in Supplementary Tables 1–6.
Abbreviations: BMI, body mass index; DDDA, Danish Diabetes Database for Adults.; T2DM, type 2 diabetes mellitus.
Men had a median body mass index (BMI) of 30.5 kg/m2 (quartiles 27.5–34.5 kg/m2), and 22.8% had a BMI above35.0 kg/m2. Women had a median BMI of 31.6 kg/m2 (quartiles 27.3–36.4 kg/m2), and 31.1% had a BMI above 35.0 kg/m2. Median waist circumference was 109 cm (quartiles 100–119 cm) for men and 104 cm (quartiles 94–114 cm) for women (Table 1).
Regarding lifestyle factors, 2.0% of women and 8.3% of men reported consuming more than the recommended weekly limit of alcohol. Less than half of the cohort (39.3%) engaged in leisure-time sport activities, while 17.9% reported always being physically inactive during leisure time (eg, reading, watching television). Among the 9039 individuals registered in the DDDA (80% of the total cohort), 18.3% were current smokers, 36.0% were former smokers, and 45.7% had never smoked (Table 1).
Characteristics at Enrollment: Comorbidities, Complications, and Medication Use
At time of DD2 enrollment, 69.4% of the cohort had a Charlson Comorbidity Index score of zero, 25.0% had a score between one and two, and 5.6% had a score of three or more (Table 2). For specific hospital-diagnosed diabetes complications, 20.7% of the cohort had a macrovascular complication (ischemic heart disease: 12.6%; heart failure: 3.8%; peripheral vascular disease: 4.7%; stroke: 5.6%), and 17.0% had at least one microvascular complication (kidney disease: 2.5%; eye disease: 8.5%; neuropathy: 7.4%). After additional ascertainment of kidney disease using routine clinical care laboratory databases, 21.1% were found to have chronic kidney disease, defined as a glomerular filtration rate below 60 mL/min/1.73 m2 or a urine albumin-creatinine ratio above 30 mg/g (Table 2).
Table 2.
Distribution of Comorbidities, Kidney Function Biomarkers, and Drug Use Among Participants in the DD2 Cohort
| Characteristics | DD2 cohort | Number with Non-Missing Data |
|---|---|---|
| Comorbidities | 11,369 (100.0%) | |
| Charlson Comorbidity Index score (based on hospital diagnoses) | ||
| 0 | 7891 (69.4%) | |
| 1–2 | 2846 (25.0%) | |
| ≥3 | 632 (5.6%) | |
| Any macrovascular complications (hospital diagnoses and procedures) | 2359 (20.7%) | |
| Ischemic heart disease | 1431 (12.6%) | |
| Heart failure | 434 (3.8%) | |
| Peripheral vascular disease | 534 (4.7%) | |
| Stroke | 640 (5.6%) | |
| Any microvascular disease (hospital diagnoses and procedures) | 1931 (17.0%) | |
| Kidney disease | 282 (2.5%) | |
| Eye disease | 966 (8.5%) | |
| Neuropathy | 844 (7.4%) | |
| Cancer excluding non-melanoma skin cancer (hospital diagnoses) | 772 (6.8%) | |
| Alcohol abuse (hospital diagnoses and treatment for elevated alcohol use) | 209 (1.8%) | |
| Kidney function (laboratory biomarkers) | ||
| eGFR, mL/min/1.73 m2 | 9808 (86.3%) | |
| <30 | 34 (0.3%) | |
| 30–60 | 701 (7.1%) | |
| >60 | 9073 (92.5%) | |
| UACR, mg/g | 8786 (77.3%) | |
| <30 | 7013 (79.8%) | |
| 30–300 | 1560 (17.8%) | |
| >300 | 213 (2.4%) | |
| Chronic kidney disease (eGFR <60 mL/min/1.73 m2 or UACR >30 mg/g) |
2264 (21.7%) | 10,417 (91.6%) |
| Drug use (prescriptions filled at community-based pharmacy) | 11,369 (100.0%) | |
| No GLD treatment | 1532 (13.5%) | |
| GLD treatment | 9837 (86.5%) | |
| GLD monotherapy | 7415 (75.4%) | |
| Non-insulin-based monotherapy | 7325 (98.8%) | |
| Insulin monotherapy | 90 (1.2%) | |
| GLD polytherapya | 2422 (24.6%) | |
| Non-insulin based polytherapy | 1831 (75.6%) | |
| Insulin based polytherapy | 591 (24.4%) | |
| Time on GLD, days before DD2 enrollment | 358 (114–884) | |
| Metformin | 9433 (83.0%) | |
| Sulfonylureas | 535 (4.7%) | |
| DPP-4 inhibitors | 933 (8.2%) | |
| SGLT-2 inhibitors | 498 (4.4%) | |
| GLP-1 receptor agonists | 818 (7.2%) | |
| Insulin | 681 (6.0%) | |
| Statins | 7759 (68.2%) | |
| Loop diuretics | 1078 (9.5%) | |
| Antihypertensives | 7952 (69.9%) | |
| ACE/ARBs | 6639 (58.4%) | |
| Calcium antagonists | 3114 (27.4%) | |
| Beta blockers | 2637 (23.2%) | |
| Thiazides | 1948 (17.1%) | |
| Potassium-sparing diuretics | 580 (5.1%) | |
| Alpha-adrenergic agents | 148 (1.3%) | |
| Platelet aggregation inhibitors | 2943 (25.9%) | |
| Antithrombotic agents | 844 (7.4%) | |
| Corticosteroids | 701 (6.2%) | |
| Antidepressants | 1658 (14.6%) | |
| Antipsychotics | 317 (2.8%) | |
| Opioids | 1812 (15.9%) |
Notes: Continuous data are presented as medians and quartiles. Count data are presented as n and proportions (%). aPolytherapy: at least one prescription each for two different GLDs or use of combination drugs. All covariate definitions are presented in Supplementary Tables 1–6.
Abbreviations: eGFR, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio; GLD, glucose-lowering drugs; SGLT-2, sodium-glucose transport protein 2; GLP1, glucagon-like peptide 1; ACE/ARBs, angiotensin-converting enzyme/angiotensin receptor blockers; DPP4, dipeptidyl peptidase 4.
At time of DD2 cohort enrollment, 86.5% of individuals were using a glucose-lowering drug, with a median duration of 358 days since initiation of the first glucose-lowering treatment (Table 2). Of these, 24.6% were receiving more than one glucose-lowering drug which primary consisted of non-insulin drugs. In total, 7.2% were using a glucagon-like peptide-1 agonist (GLP-1RA) and 4.4% were using a sodium-glucose cotransporter-2 inhibitor (SGLT2i) at enrollment (Table 2).
Usage of cardiovascular and nephroprotective drugs beyond glucose lowering medications was relatively high: 68.2% were receiving statins; 69.9% were receiving antihypertensive agents; and 58.4% were receiving an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker. As well, 14.6% were using antidepressants and 15.9% were using opioids (Table 2).
Characteristics at Enrollment: Biomarkers and Genotyping
The most recent HbA1c level before cohort enrollment was near the clinical cut-off for defining diabetes: the median HbA1c level was 48.0 mmol/mol (quartiles 43.0–54.0 mmol/mol). However, prior to administration of the first glucose-lowering drug, the median HbA1c level was 54.0 mmol/mol (quartiles 50.0–72.0 mmol/mol) (Table 3). The median low-density lipoprotein (LDL) cholesterol level was 2.2 mmol/L (quartiles 1.7–2.9 mmol/L), and 27.4% of the cohort had LDL cholesterol levels above 2.6 mmol/L. The median triglyceride level was 1.7 mmol/L (quartiles 1.2–2.4 mmol/L). Additional 26 biomarkers (eg, hsCRP, TNF-α, adiponectin, leptin) have been analyzed for research studies11,19,21,42 nested within the DD2 cohort (the full list of biomarkers is shown in Supplementary Table 7). As of 2023, genotyping has been performed on 10,162 individuals, with 9422 procedures passing quality control.
Table 3.
Selected Biomarker Values for 11,369 DD2 Cohort Participants at Enrollment, Assessed from Tests Performed as Part of Routine Clinical Care or from Dedicated Analyses of Biobank Samples
| Biomarkers Measured in Routine Clinical Care | Biomarker Level | Number with Non-Missing Data |
|---|---|---|
| HbA1c, mmol/mol | 48.0 (43.0–54.0) | 10,444 (91.9%) |
| HbA1c before GLD treatment, mmol/mol | 54.0 (49.0–72.0) | 7184 (63.2%) |
| LDL cholesterol, mmol/L | 2.2 (1.7–2.9) | 9672 (85.1%) |
| Total cholesterol, mmol/L | 4.3 (3.6–5.0) | 9362 (82.3%) |
| Triglycerides, mmol/L | 1.7 (1.2–2.4) | 9839 (86.5%) |
| HDL cholesterol, mmol/L | 1.2 (1.0–1.4) | 9372 (82.4%) |
| eGFR, mL/min/1.73 m2 | 90.4 (76.9–100.2) | 9808 (86.3%) |
| Albumin-creatinine ratio, mg/g | 9.1 (5.0–23.0) | 8786 (77.3%) |
| Examples of biomarkers analyzed in DD2 biobank samples | ||
| Blood test available in biobank | - | 10,796 (95.0%) |
| Fasting blood test available in biobank | - | 8778 (77.9%) |
| Fasting serum C-peptide, pmol/L | 1140.0 (855.2–1521.0) | 7776 (68.4%) |
| Fasting plasma glucose, mmol/L | 7.2 (6.4–8.3) | 7767 (68.3%) |
| Glutamic acid decarboxylase antibodies ≥20 IU/mL | 165 (2.9%) | 5756 (50.6%) |
| MBL, µg/L | 734.7 (225.0–1634.1) | 7287 (64.1%) |
| hsCRP, mg/L | 2.0 (0.9–4.5) | 9663 (85.0%) |
Notes: Continuous data are presented as medians and quartiles. Count data are presented as n and proportions (%). All covariate definitions are presented in Supplementary Tables 1–6.
Abbreviations: eGFR, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio; LDL, low density lipoprotein; HDL, high density lipoprotein; hsCRP, high sensitivity C-reactive protein; MBL, mannose-binding lectin.
Post-Enrollment Longitudinal Follow-Up Data: Rates of Mortality and Cardiovascular Disease Outcomes
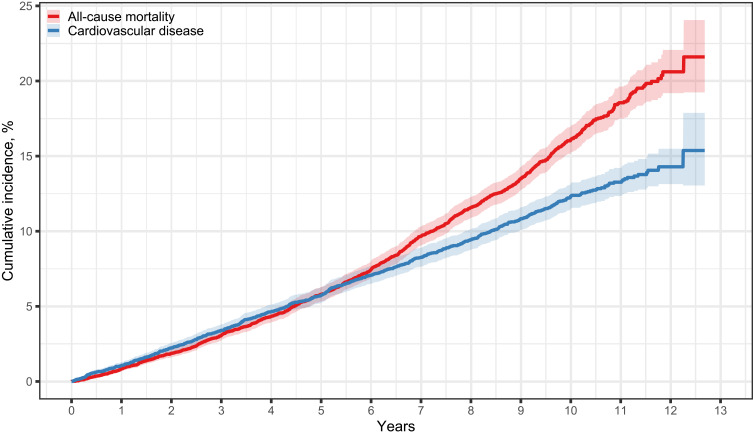
During a median follow-up period of 7.9 years, 1361 individuals died. This corresponds to a mortality rate of 17.6 events per 1000 person-years, a 5-year mortality risk of 5.8%, and a 10-year mortality risk of 16.1% (Supplementary Table 8 and Figure 3). Among the 9572 individuals without a prior CVD diagnosis at enrollment, 870 developed incident CVD during follow-up. This represented an incidence rate of 13.8 events per 1000 person-years. After considering the competing risk of death, the 5-year risk of incident CVD was 5.7% and the 10-year risk was 12.3% (Supplementary Table 8 and Figure 3).
Figure 3.
Risk of mortality and incident cardiovascular disease after enrollment in the DD2 cohort. Incident cardiovascular disease events include acute myocardial infarction, unstable angina, heart failure, coronary artery bypass grafting/percutaneous coronary intervention procedures, stroke, and cerebral thrombolysis/thrombectomy procedures. Patients were excluded from the analysis of cardiovascular disease if they had a preexisting hospital diagnosis or procedure code before baseline (N=1797). The Kaplan Meier estimator was used to depict all-cause mortality, and the Aalen-Johansen estimator was used for cardiovascular disease, taking the competing risk of death into consideration.
Discussion
The DD2 cohort has undergone an important expansion since the latest cohort description in 2018. It now includes a larger number of participants, additional data sources, and linkage to a wider range of health and administrative registries. Furthermore, the cohort database holds results of several biomarkers and genotypes generated in nested research studies. As part of Denmark’s unique network of health databases, the DD2 cohort stands out as one of the most detailed cohorts available globally for tracking the clinical course of patients with newly diagnosed T2DM.
Comparison with Other European T2D Data Sources
A few diabetes cohorts in Europe are comparable to the DD2 cohort, including the Swedish All New Diabetics in Scania cohort (ANDIS, N=26,962) and the Genetics of Diabetes Audit and Research in Tayside, Scotland (GODARTS, N=10,149) cohort.43,44 Like DD2, these regional cohorts enroll individuals from general practices and hospital outpatient clinics. They also collect and store biological samples, and baseline data are linked to electronic health records for prospective follow-up.43,44 In contrast to ANDIS and DD2, the GODARTS cohort includes matched healthy individuals allowing comparison of health outcomes between individuals with and without diabetes.
The German Diabetes Study45 and the Maastricht Study represent two other similar European diabetes cohorts.46 The German Diabetes Study is a longitudinal cohort study of individuals with diabetes enrolled from German hospital outpatient clinics, media campaigns, and general practices (N=1105). Unlike DD2, this study also includes dedicated extensive follow-up examinations.45 The Maastricht Study of individuals with and without diabetes (N=9187) recruits individuals through media campaigns, municipal registries, and the regional Diabetes Patient Registry.46 Other diabetes cohorts in Europe – though not specific to T2DM – include the Swedish Scania Diabetes Registry (N=7400), which recruited individuals with diabetes from hospitals in Scania between 1996 and 2009;47 the All New Diabetics in Uppsala registry established in 2012 (N=844);48 and the Diabetes Registry Vaasa (N=5107) in Western Finland.49
Comparison of Mortality and CVD Rates
The overall mortality rate in our study cohort from 2010 to 2023 aligns well with mortality observed in a Danish study of all first-time initiators of glucose-lowering drugs between 1995 and 2018 (5-year risk: approximately 5%),50 suggesting that our cohort is not skewed towards particularly healthy individuals with T2DM. However, mortality rates in the cohort were slightly lower than those observed in Scotland among individuals with a first-time diabetes diagnosis made in a general practice or hospital setting from 2004 to 2013 (18.1 events per 1000 person-years; unknown median follow-up time).51 Other studies have estimated mortality rates mainly in mixed cohorts of individuals with new or prevalent T2DM. This may hamper direct comparison with our cohort of individuals with newly diagnosed T2DM.52–55
There is evidence that death rates among individuals with T2DM have declined in western countries over recent decades.50,52,55,56 This improvement is likely attributable to several factors: an increase in T2DM screening activity based on HbA1c levels; updated clinical guidelines emphasizing early intensive therapeutic interventions for patients with T2DM and an accompanying decline in “watchful waiting” focusing on lifestyle changes alone; and improved multifactorial management of cardiovascular risk factors for high-risk patients with T2DM.50,52,55,56 Consistent with improved risk factor management, the 10-year risk of CVD was relatively low (12.3%) in the DD2 cohort. This is comparable to the results of a recent population-based cohort study of all individuals with T2DM in Denmark between 2006 and 2013, which reported a 10-year risk of 12.0% for incident CVD.57 This suggests that participants in the DD2 cohort may be representative of average individuals with T2DM in Denmark.
Present Contribution of the DD2 Cohort to T2DM Research
To date, the DD2 cohort has contributed to research in several areas. For example, observational studies based on DD2 data have focused on risk factors and prognosis for diabetic neuropathy;22,58–60 the association between low-grade inflammation markers and risk of CVD,21,61 characteristics of individuals with early-onset T2DM,62,63 and the impact of birth weight on T2DM characteristics and outcomes later in life.11
The ongoing DD2 sub-segmentation project addresses the heterogeneity in the clinical presentation of T2DM. Current evidence suggests that T2DM can be categorized into subgroups based on genetic risk profiles and metabolites measured in routine clinical care.6,64,65 The currently best known example, originating from Sweden, uses glutamic acid decarboxylase antibodies, age at diagnosis, BMI at diagnosis, HbA1c level, and HOMA-2 estimates of insulin resistance and beta-cell function to categorize patients into the following groups: autoimmune diabetes, insulin-deficient diabetes, insulin-resistant diabetes, obesity-related diabetes, and age-related diabetes.43,64 These subgroups manifest different patterns of clinical features, disease progression, and onset of complications.6,64,66 The DD2 sub-segmentation project and prior DD2 studies have used indices of insulin sensitivity and beta-cell function to define three T2DM subgroups: an insulinopenic subgroup (high insulin sensitivity, low beta-cell function), a classical subgroup (low insulin sensitivity, low beta-cell function), and a hyperinsulinemic subgroup (low insulin sensitivity, high beta-cell function).8–10,67 By directly comparing the two subgroup classifications described above, another DD2-based study found a high degree of overlap between the Swedish insulin-resistant subgroup and the DD2 hyperinsulinemic subgroup (70%), while the Swedish insulin-deficient, obesity-related, and age-related subgroup all had a substantial overlap with the DD2 classical subgroup (>70%).8 Consensus regarding the standard definition of the T2DM subgroups is currently lacking, and the quality of evidence supporting the clinical use of these T2DM subclassification approaches is moderate.6,64 Future analyses of the DD2 cohort may aid in understanding the heterogeneity and secondary prevention of complications in individuals with T2DM.
Weaknesses of the DD2 Cohort
The DD2 cohort has limitations. First, while most general practices in Denmark have been invited to recruit T2DM patients for the DD2 project, only 22% of practices are currently participating. Most clinics are in the South Denmark Region, the Central Denmark Region, and the Capital Region. Second, while the DD2 project aims to recruit participants with recent-onset T2DM, persons with T2DM duration of several years have been allowed to participate between 2012 and 2016. However, they account for only a small part of the total cohort, in which the median T2DM duration is 1.3 years (quartiles 0.4–2.9 years). Furthermore, studies have suggested that first-time initiators of glucose-lowering drugs in the general population have demographic and clinical characteristics similar to those of participants in the DD2 cohort.68 Third, despite the availability of many data sources and the high completeness of primary data collected at DD2 enrollment, there are some missing baseline data in the cohort because the external data sources that DD2 relies on do not have records for all cohort participants. Assuming that these data are missing at random, researchers may use multiple imputation or other statistical methods to account for possible bias when conducting a complete case analysis.69 Fourth, a detailed collection of data on socio-behavioral factors is lacking. Changes in Danish national recommendations for alcohol consumption issued by the Danish Health Authority have not been implemented in the DD2 baseline questionnaire.70 Despite these recommendations have changed over the years, the registration in DD2 still match the 2010 recommendations. Although clinicians often perceive smoking, alcohol consumption, poor diet, lack of physical activity, and obesity as key risk factors for many chronic diseases, including T2DM, these factors often arise from a range of upstream psychosocial determinants. These include stress, anxiety, depression, poor sleep, social isolation, difficult working conditions, low education and health literacy, as well as political determinants such as health care organization, housing conditions, employment, economy security, alcohol recommendations, and food prices.71–74 The DD2 study group is currently working to secure data collection of several of these key variables, and an improved baseline questionnaire is under development.
Conclusions
The interlinked DD2 cohort and biobank is a unique dataset that has already improved our understanding of diabetes complications among individuals with newly diagnosed T2DM. Our data provide evidence that in Denmark - a Scandinavian country aiming to promote health equity through tax-financed universal health care - more than one-third of individuals with new T2DM already have established cardiorenal complications at T2DM diagnosis, with obesity being a major underlying factor. Although use of traditional CVD preventive therapies was relatively high, one out of five DD2 cohort participants died within 12 years after enrollment.
Nested in the large network of Danish health and social registries, the DD2 cohort provides a unique foundation for future cost-effective clinical epidemiological research with complete life-long follow-up throughout the course of T2DM disease. The DD2 cohort and biobank are thus expected to be used extensively in coming years for conducting T2DM research at the highest international level. Findings from DD2 will improve our understanding of the progression and prevention of complications among individuals with newly diagnosed T2DM.
Acknowledgments
We are very grateful to all physicians willing to recruit participants into the DD2 cohort and to all DD2 participants. We thank Klara Berencsi, MD, PhD, and all biostatisticians at the Department of Clinical Epidemiology, Aarhus University and Aarhus University Hospital, who have organized, coded, and maintained the linked DD2 cohort over many years.
Funding Statement
The Danish Centre for Strategic Research in Type 2 Diabetes Project is supported by the Danish Agency for Science (grant number: 09-067009, 10-079102, 09-075724), the Danish Health Authority, the Danish Medicines Agency, the Danish Diabetes Association, and Novo Nordisk Foundation (grant number: NNF17SA0030364, NNF16SA0024768, NNF20OC0063292)., NNF23SA0088557). Project partners are listed on the website www.DD2.dk. This work was also supported by a grant from the Novo Nordisk Foundation (NNF23SA0084103). Furthermore, this work has been supported partly by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation.
Data Sharing Statement
Data are available for research upon request to the DD2 Scientific Advisory Board, and/or the Steering Group of the NNF20OC0063292 grant (see below), all within the framework of Danish data protection legislation and required permissions from authorities.
Ethics Approval
The Danish Regional Ethical Committee on Health Research for Southern Denmark (record no. S-20100082) and the Danish Data Protection Agency (record nos. 2008-58-0035 and 2016-051-000001/2514) approved the DD2 study. Participants provided signed written informed consent before enrollment.
Collaboration
The DD2 Project has a Scientific Advisory Board (SAB) with members from general practices/hospital research units in all regions of Denmark. The SAB strongly encourages national and international collaboration. A Board of Directors (BoD) oversees DD2. The members of the BoD are the CEOs of the five Danish Steno Diabetes Centers, with the CEO of Steno Diabetes Center Odense serving as Chair. More information regarding the administration and supervision of the DD2 cohort can be found at the DD2 website www.dd2.dk. A list of prior publications using DD2 data is available at: www.dd2.dk/forskning/videnskabelige-publikationer and www.dd2.dk/forskning/projektoversigt.
Author Contributions
F.P.B.K., R.W.T., S.K.N., and H.T.S. conceived the idea for this Cohort Profile Paper. S.K.N. performed all statistical analyses. F.P.B.K., S.K.N., R.W.T., and H.T.S. wrote the first paper draft. The DD2 project was initiated by H.B.N., H.T.S., J.S.C., J.R., and A.V. J.S.C. passed away in 2015. J.S.C was one of the leading researchers in conception and design of the DD2 Project. I.B. is in charge of storage and analyses of biological samples. A.V. is PI and C.B. project manager of the NNF20OC0063292 grant entitled “Closing in on Sub-Segmentation in Type 2 Diabetes in the Danish Nationwide DD2 Cohort” providing and gating MSD circulating biomarkers, birth-related data from midwife paper records, and genotype data on 6000 participants. M.K.A. and T.H. are responsible for the generation, quality control, and management of the genetic data. J.S.N., K.H., and R.W.T. handle the daily management of DD2 data and biobank. S.K.N. is responsible for overall data management and linkage of the DD2 cohort to secondary registry data. All authors contributed to the interpretation of the data. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. F.P.B.K., R.W.T., S.K.N., and H.T.S. had full access to all the data in the study and take responsibility for the integrity and the accuracy of the data analysis of the current publication.
Disclosure
D.H.C reports grants from the Danish Diabetes and Endocrine Academy, outside the submitted work. I.B. is a partner in the DD2 project and received funding from DD2 to host and manage the DD2 Biobank. P.V. is head of research at Steno Diabetes Center North Denmark funded by an unrestricted grant from the Novo Nordisk Foundation. M.H.O. reports personal fees from Novo Nordic A/S, Teva A/S, AstraZeneca A/S, and Boehringer & Ingelheim, outside the submitted work. C.B. is a stock owner of Novo Nordisk. The Department of Clinical Epidemiology, Aarhus University and Aarhus University Hospital, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of those studies have any relation to the present study. The authors report no other conflicts of interest in this work.
References
- 1.Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet. 2022;400(10365):1803–1820. doi: 10.1016/S0140-6736(22)01655-5 [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabet Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetestal Diabetesforeningen; 2023. Available from: https://www.diabetestal.nu/. Accessed August 27, 2024.
- 4.Kjellberg J, Tikkanen CK, Bagger M, Gæde P. Short-term societal economic burden of first-incident type 2 diabetes-related complications - a nationwide cohort study. Expert Rev Pharmacoecon Outcomes Res. 2020;20(6):577–586. doi: 10.1080/14737167.2020.1837626 [DOI] [PubMed] [Google Scholar]
- 5.Laurberg T, Graversen SB, Sandbæk A, Wild SH, Vos RC, Støvring H. Trends in cause-specific mortality among people with type 2 and type 1 diabetes from 2002 to 2019: a Danish population-based study. Lancet Reg Health Eur. 2024;41:100909. doi: 10.1016/j.lanepe.2024.100909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobias DK, Merino J, Ahmad A, et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nature Med. 2023;29(10):2438–2457. doi: 10.1038/s41591-023-02502-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sørensen HT, Friborg S, Rungby J, Christensen JS, Vaag A, Beck-Nielsen H. The Danish national type 2 diabetes cohort - The DD2 study. Clin Epidemiol. 2012;4(Suppl 1):1–5. doi: 10.2147/CLEP.S31104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen DH, Nicolaisen SK, Ahlqvist E, et al. Type 2 diabetes classification: a data-driven cluster study of the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) cohort. BMJ Open Diabetes Res Care. 2022;10(2):e002731. doi: 10.1136/bmjdrc-2021-002731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stidsen JV, Christensen DH, Henriksen JE, et al. Risk of cardiovascular events associated with pathophysiological phenotypes of type 2 diabetes. Eur J Endocrinol. 2022;187(2):279–291. doi: 10.1530/EJE-22-0020 [DOI] [PubMed] [Google Scholar]
- 10.Stidsen JV, Henriksen JE, Olsen MH, et al. Pathophysiology-based phenotyping in type 2 diabetes: a clinical classification tool. Diabetes Metab Res Rev. 2018;34(5):e3005. doi: 10.1002/dmrr.3005 [DOI] [PubMed] [Google Scholar]
- 11.Hansen AL, Thomsen RW, Brøns C, et al. Birthweight is associated with clinical characteristics in people with recently diagnosed type 2 diabetes. Diabetologia. 2023;66(9):1680–1692. doi: 10.1007/s00125-023-05936-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen DH, Nicolaisen SK, Berencsi K, et al. Danish Centre for Strategic Research in Type 2 Diabetes (DD2) project cohort of newly diagnosed patients with type 2 diabetes: a cohort profile. BMJ Open. 2018;8(4):e017273. doi: 10.1136/bmjopen-2017-017273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen JS, Thomsen RW, Steffensen C, Christiansen JS. The Danish Centre for Strategic Research in Type 2 Diabetes (DD2) study: implementation of a nationwide patient enrollment system. Clin Epidemiol. 2012;4(Suppl 1):27–36. doi: 10.2147/CLEP.S30838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steffensen C, Thomsen RW, Vaag A, et al. The Danish Centre for Strategic Research in Type 2 Diabetes (DD2) Project: rationale and planned nationwide studies of genetic predictors, physical exercise, and individualized pharmacological treatment. Clin Epidemiol. 2012;4(Suppl 1):7–13. doi: 10.2147/CLEP.S30188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomsen RW, Friborg S, Nielsen JS, Schroll H, Johnsen SP. The Danish centre for strategic research in type 2 Diabetes (DD2): organization of diabetes care in Denmark and supplementary data sources for data collection among DD2 study participants. Clin Epidemiol. 2012;4(Suppl 1):15–19. doi: 10.2147/CLEP.S30082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt M, Pedersen L, Sorensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 18.Christensen H, Nielsen JS, Sørensen KM, Melbye M, Brandslund I. New national biobank of the Danish center for strategic research on type 2 diabetes (DD2). Clin Epidemiol. 2012;4:37–42. doi: 10.2147/CLEP.S33042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gedebjerg A, Thomsen RW, Kjaergaard AD, et al. Mannose-binding lectin and risk of infections in type 2 diabetes: a Danish cohort study. J Diabetes Complications. 2021;35(5):107873. doi: 10.1016/j.jdiacomp.2021.107873 [DOI] [PubMed] [Google Scholar]
- 20.Thuesen ACB, Jensen RT, Maagensen H, et al. Identification of pathogenic GCK variants in patients with common type 2 diabetes can lead to discontinuation of pharmacological treatment. Mol Genet Metab Rep. 2023;35:100972. doi: 10.1016/j.ymgmr.2023.100972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gedebjerg A, Bjerre M, Kjaergaard AD, et al. CRP, C-peptide, and risk of first-time cardiovascular events and mortality in early type 2 diabetes: a Danish cohort study. Diabetes Care. 2023;46(5):1037–1045. doi: 10.2337/dc22-1353 [DOI] [PubMed] [Google Scholar]
- 22.Gylfadottir SS, Christensen DH, Nicolaisen SK, et al. Diabetic polyneuropathy and pain, prevalence, and patient characteristics: a cross-sectional questionnaire study of 5514 patients with recently diagnosed type 2 diabetes. Pain. 2019;161(3):574–583. doi: 10.1097/j.pain.0000000000001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ried-Larsen M, Thomsen RW, Berencsi K, et al. Implementation of interval walking training in patients with type 2 diabetes in Denmark: rationale, design, and baseline characteristics. Clin Epidemiol. 2016;8:201–209. doi: 10.2147/CLEP.S97303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stidsen JV, Nielsen JS, Henriksen JE, et al. Protocol for the specialist supervised individualised multifactorial treatment of new clinically diagnosed type 2 diabetes in general practice (IDA): a prospective controlled multicentre open-label intervention study. BMJ Open. 2017;7(12):e017493. doi: 10.1136/bmjopen-2017-017493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domazet SL, Tarp J, Thomsen RW, et al. Accelerometer-derived physical activity and sedentary behaviors in individuals with newly diagnosed type 2 diabetes: a cross-sectional study from the Danish nationwide DD2 cohort. Front Sports Act Living. 2023;4:1089579. doi: 10.3389/fspor.2022.1089579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valentiner LS, Ried-Larsen M, Karstoft K, et al. Long-term effect of smartphone-delivered Interval Walking Training on physical activity in patients with type 2 diabetes: protocol for a parallel group single-blinded randomised controlled trial. BMJ Open. 2017;7(4):e014036. doi: 10.1136/bmjopen-2016-014036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jørgensen ME, Kristensen JK, Reventlov Husted G, Cerqueira C, Rossing P. The Danish adult diabetes registry. Clin Epidemiol. 2016;8:429–434. doi: 10.2147/CLEP.S99518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pottegard A, Schmidt SAJ, Wallach-Kildemoes H, Sorensen HT, Hallas J, Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;46(3):798–798f. doi: 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arendt JFH, Hansen AT, Ladefoged SA, Sørensen HT, Pedersen L, Adelborg K. Existing data sources in clinical epidemiology: laboratory information system databases in Denmark. Clin Epidemiol. 2020;12:469–475. doi: 10.2147/CLEP.S245060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7_suppl):42–45. doi: 10.1177/1403494810393562 [DOI] [PubMed] [Google Scholar]
- 32.Munk-Jørgensen P, Mortensen PB. The Danish psychiatric central register. Dan Med Bull. 1997;44(1):82–84. [PubMed] [Google Scholar]
- 33.Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39(7 Suppl):54–57. doi: 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- 34.Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7 Suppl):91–94. doi: 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- 35.Petersson F, Baadsgaard M, Thygesen LC. Danish registers on personal labour market affiliation. Scand J Public Health. 2011;39(7 Suppl):95–98. doi: 10.1177/1403494811408483 [DOI] [PubMed] [Google Scholar]
- 36.Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7 Suppl):103–105. doi: 10.1177/1403494811405098 [DOI] [PubMed] [Google Scholar]
- 37.Hjollund NH, Larsen FB, Andersen JH. Register-based follow-up of social benefits and other transfer payments: accuracy and degree of completeness in a Danish interdepartmental administrative database compared with a population-based survey. Scand J Public Health. 2007;35(5):497–502. doi: 10.1080/14034940701271882 [DOI] [PubMed] [Google Scholar]
- 38.Andersen JS, Olivarius Nde F, Krasnik A. The Danish national health service register. Scand J Public Health. 2011;39(7 Suppl):34–37. doi: 10.1177/1403494810394718 [DOI] [PubMed] [Google Scholar]
- 39.Bliddal M, Broe A, Pottegård A, Olsen J, Langhoff-Roos J. The Danish medical birth register. Eur J Epidemiol. 2018;33(1):27–36. doi: 10.1007/s10654-018-0356-1 [DOI] [PubMed] [Google Scholar]
- 40.Pottegård A, Kristensen KB, Reilev M, et al. Existing Data Sources in Clinical Epidemiology: the Danish COVID-19 Cohort. Clin Epidemiol. 2020;12:875–881. doi: 10.2147/CLEP.S257519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011;39(7 Suppl):26–29. doi: 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 42.Domazet SL, Olesen TB, Stidsen JV, et al. Low-grade inflammation in persons with recently diagnosed type 2 diabetes: the role of abdominal adiposity and putative mediators. Diabetes Obes Metab. 2024;26:2092–2101. doi: 10.1111/dom.15514 [DOI] [PubMed] [Google Scholar]
- 43.Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369. doi: 10.1016/S2213-8587(18)30051-2 [DOI] [PubMed] [Google Scholar]
- 44.Hébert HL, Shepherd B, Milburn K, et al. Cohort profile: genetics of diabetes audit and research in Tayside Scotland (GoDARTS). Int J Epidemiol. 2017;47(2):380–381j. doi: 10.1093/ije/dyx140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szendroedi J, Saxena A, Weber KS, et al. Cohort profile: the German Diabetes Study (GDS). Cardiovas Diabetol. 2016;15(1):59. doi: 10.1186/s12933-016-0374-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schram MT, Sep SJ, van der Kallen CJ, et al. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol. 2014;29(6):439–451. doi: 10.1007/s10654-014-9889-0 [DOI] [PubMed] [Google Scholar]
- 47.Scania Diabetes Registry. Swedish national data service. Available from: https://snd.gu.se/en/catalogue/dataset/ext0074-1. Accessed August 27, 2024.
- 48.ESTRID - Epidemiological study of risk factors for LADA and type 2 diabetes. Karolinska Institutet; 2024. Available from: https://ki.se/en/imm/study-design. Accessed August 27, 2024.
- 49.DIREVA. Lund University; 2024. Available from: https://www.ludc.lu.se/resources/cohorts/direva. Accessed August 27, 2024.
- 50.Knudsen JS, Knudsen SS, Hulman A, et al. Changes in type 2 diabetes incidence and mortality associated with introduction of HbA1c as diagnostic option: a Danish 24-year population-based study. Lancet Reg Health Eur. 2022;14:100291. doi: 10.1016/j.lanepe.2021.100291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Read SH, Kerssens JJ, McAllister DA, et al. Trends in type 2 diabetes incidence and mortality in Scotland between 2004 and 2013. Diabetologia. 2016;59(10):2106–2113. doi: 10.1007/s00125-016-4054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearson-Stuttard J, Bennett J, Cheng YJ, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021;9(3):165–173. doi: 10.1016/S2213-8587(20)30431-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norhammar A, Bodegård J, Nyström T, Thuresson M, Eriksson JW, Nathanson D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose-lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006–2013. Diabetologia. 2016;59(8):1692–1701. doi: 10.1007/s00125-016-3971-y [DOI] [PubMed] [Google Scholar]
- 54.Carstensen B, Rønn PF, Jørgensen ME. Prevalence, incidence and mortality of type 1 and type 2 diabetes in Denmark 1996–2016. BMJ Open Diabetes Res Care. 2020;8(1):e001071. doi: 10.1136/bmjdrc-2019-001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391(10138):2430–2440. doi: 10.1016/S0140-6736(18)30314-3 [DOI] [PubMed] [Google Scholar]
- 56.Gyldenkerne C, Knudsen JS, Olesen KKW, et al. Nationwide trends in cardiac risk and mortality in patients with incident type 2 diabetes: a Danish cohort study. Diabetes Care;2021. dc210383. doi: 10.2337/dc21-0383 [DOI] [PubMed] [Google Scholar]
- 57.Gyldenkerne C, Mortensen MB, Kahlert J, et al. 10-year cardiovascular risk in patients with newly diagnosed type 2 diabetes mellitus. J Am Coll Cardiol. 2023;82(16):1583–1594. doi: 10.1016/j.jacc.2023.08.015 [DOI] [PubMed] [Google Scholar]
- 58.Khan KS, Christensen DH, Nicolaisen SK, et al. Falls and fractures associated with type 2 diabetic polyneuropathy; a cross-sectional nationwide questionnaire study. J Diabetes Investig. 2021;12(10):1827–1834. doi: 10.1111/jdi.13542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bjerg L, Nicolaisen SK, Christensen DH, et al. Diabetic polyneuropathy early in type 2 diabetes is associated with higher incidence rate of cardiovascular disease: results from two Danish cohort studies. Diabetes Care. 2021;44(7):1714–1721. doi: 10.2337/dc21-0010 [DOI] [PubMed] [Google Scholar]
- 60.Christensen DH, Knudsen ST, Gylfadottir SS, et al. Metabolic factors, lifestyle habits, and possible polyneuropathy in early type 2 diabetes: a nationwide study of 5249 patients in the Danish centre for strategic research in type 2 diabetes (DD2) cohort. Diabetes Care. 2020;43(6):1266–1275. doi: 10.2337/dc19-2277 [DOI] [PubMed] [Google Scholar]
- 61.Gedebjerg A, Bjerre M, Kjaergaard AD, et al. Mannose-binding lectin and risk of cardiovascular events and mortality in type 2 diabetes: a Danish cohort study. Diabetes Care. 2020;43(9):2190–2198. doi: 10.2337/dc20-0345 [DOI] [PubMed] [Google Scholar]
- 62.Bo A, Thomsen RW, Nielsen JS, et al. Early-onset type 2 diabetes: age gradient in clinical and behavioural risk factors in 5115 persons with newly diagnosed type 2 diabetes-Results from the DD2 study. Diabetes Metab Res Rev. 2018;34(3). doi: 10.1002/dmrr.2968 [DOI] [PubMed] [Google Scholar]
- 63.Bo A, Pouwer F, Juul L, Nicolaisen SK, Maindal HT. Prevalence and correlates of diabetes distress, perceived stress and depressive symptoms among adults with early-onset Type 2 diabetes: cross-sectional survey results from the Danish DD2 study. Diabet Med. 2020;37(10):1679–1687. doi: 10.1111/dme.14087 [DOI] [PubMed] [Google Scholar]
- 64.Herder C, Roden M. A novel diabetes typology: towards precision diabetology from pathogenesis to treatment. Diabetologia. 2022;65(11):1770–1781. doi: 10.1007/s00125-021-05625-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet. 2014;383(9922):1084–1094. doi: 10.1016/S0140-6736(13)62219-9 [DOI] [PubMed] [Google Scholar]
- 66.Ahlqvist E, Prasad RB, Groop L. Subtypes of type 2 diabetes determined from clinical parameters. Diabetes. 2020;69(10):2086–2093. doi: 10.2337/dbi20-0001 [DOI] [PubMed] [Google Scholar]
- 67.Kristensen FPB, Christensen DH, Callaghan BC, et al. The prevalence of polyneuropathy in type 2 diabetes subgroups based on HOMA2 Indices of β-cell function and insulin sensitivity. Diabetes Care. 2023;46(8):1546–1555. doi: 10.2337/dc23-0079 [DOI] [PubMed] [Google Scholar]
- 68.Thomsen RW, Baggesen LM, Svensson E, et al. Early glycaemic control among patients with type 2 diabetes and initial glucose-lowering treatment: a 13-year population-based cohort study. Diabetes Obes Metab. 2015;17(8):771–780. doi: 10.1111/dom.12484 [DOI] [PubMed] [Google Scholar]
- 69.Hughes RA, Heron J, Sterne JAC, Tilling K. Accounting for missing data in statistical analyses: multiple imputation is not always the answer. Int J Epidemiol. 2019;48(4):1294–1304. doi: 10.1093/ije/dyz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anbefalinger om alkohol. The Danish health authority; 2024. Available from: https://sst.dk/da/borger/en-sund-hverdag/alkohol/anbefalinger-om-alkohol. Accessed August 27, 2024.
- 71.Walker AF, Graham S, Maple-Brown L, et al. Interventions to address global inequity in diabetes: international progress. Lancet. 2023;402(10397):250–264. doi: 10.1016/S0140-6736(23)00914-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toft Sørensen H, Bredahl Kristensen FP. Cardiovascular diseases and health inequalities in Europe—a pressing public health challenge. Lancet Reg Health. 2023;33:100722. doi: 10.1016/j.lanepe.2023.100722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hill-Briggs F, Fitzpatrick SL. Overview of social determinants of health in the development of diabetes. Diabetes Care. 2023;46(9):1590–1598. doi: 10.2337/dci23-0001 [DOI] [PubMed] [Google Scholar]
- 74.Hunter DJ. The complementarity of public health and medicine - achieving “the highest attainable standard of health”. N Engl J Med. 2021;385(6):481–484. doi: 10.1056/NEJMp2102550 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available for research upon request to the DD2 Scientific Advisory Board, and/or the Steering Group of the NNF20OC0063292 grant (see below), all within the framework of Danish data protection legislation and required permissions from authorities.