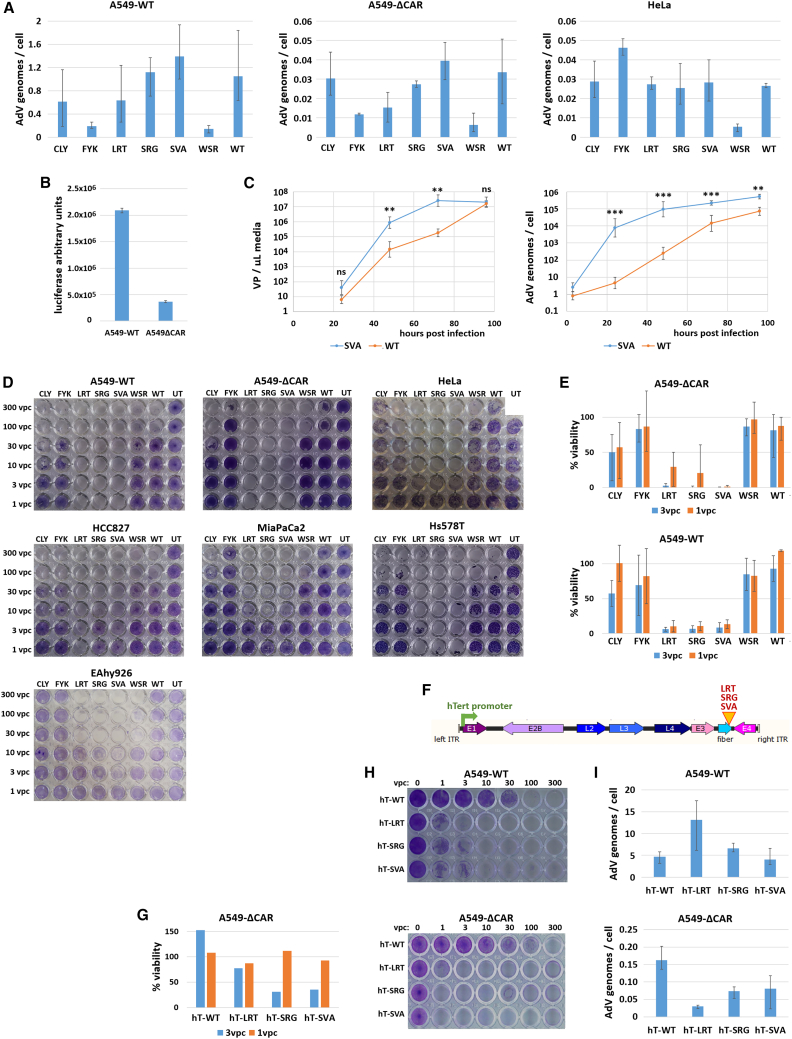
Figure 3.
ADEVO-selected variants show improved cell lysis and DNA replication but not increased infectivity
(A) ADEVO-selected variants do not display modified tropism. A549-WT, A549-ΔCAR, and HeLa cells were infected by 10 vector particles per cell (vpc) of HAdV-C5-WT (WT) or ADEVO-selected variants. At 3 h post infection (hpi), internalized AdV genomes were quantified. ADEVO-selected variants do not display preferential infection of the A549-ΔCAR cells in which they were selected compared with other cell lines, while HAdV-C5-WT infected A549-ΔCAR cells efficiently. n = 6, two independent experiment repeats, error bars: maximum-minimum. (B) A549-ΔCAR cells are permissive to non-modified HAdV-C5 infection. A549-ΔCAR and A549-WT cells were infected at 20 vpc by a luciferase-expressing HAdV-C5 vector. Luciferase luminescence was quantified at 24 hpi, showing relatively strong viral transgene expression in A549-ΔCAR cells. n = 2. (C) The SVA variant replicates faster than HAdV-C5-WT. A549-WT cells were infected with 10 vpc of HAdV-C5-WT (WT) or SVA variant. At regular intervals, culture supernatant was collected and excreted VPs were quantified. Alternatively, cells were harvested, washed thoroughly, and intracellular AdV genomes were quantified. n = 6, two independent repeats, data points: geometric mean ± standard deviation. SVA and HAdV-C5-WT titers at each time point were normalized on the respective internalized genome titers at 3 hpi and compared by Welch T test (Mann-Whitney U tests confirmed the significance in all cases where t test indicated it). ns, p > 0.05. ∗∗0.01 > p > 0.001. ∗∗∗0.001 > p. Two-sided p values. (D) The LRT, SRG, and SVA variants lyse efficiently a wide array of cancer cell lines. Cancer cell lines were infected by HAdV-C5-WT or ADEVO-selected variants at various multiplicities of infection ranging from 1 to 300 vpc. Several days later (3 days post infection [dpi] for A549-WT cells, 5 dpi for HCC827 and Hs578T cells, 6 dpi for A549-ΔCAR, HeLa and MiaPaCa2 cells and 7 dpi for EAhy926 cells), live cells were stained with crystal violet. UT, untransduced cells; WT, HAdV-C5-WT. Two to four experiment repeats were performed; representative pictures are shown. (E) CCK8 quantification of infected A549-ΔCAR and A549-WT cells viability. Before crystal violet staining (Figure 3D), the viability of cells infected with 3 vpc or 1 vpc was quantified and normalized on the viability of uninfected cells cultivated in the same conditions. n = 3, three independent experiment repeats, error bars: maximum-minimum. (F) Schematic representation of the generated replication-selected vectors carrying the LRT, SRG, or SVA fiber inserts as well as a hTert promoter controlling E1 gene expression. (G) CCK8 quantification of infected A549-ΔCAR and A549-WT cells viability. Before crystal violet staining (Figure 3H), the viability of cells infected with 3 vpc or 1 vpc was quantified and normalized on the viability of uninfected cells cultivated in the same conditions. n = 1. hT-WT, HAdV-C5-hTert with WT fiber; hT-LRT/SRG/SVA, HAdV-C5-hTert with fiber insert. (H) The replication-selective variants lyse cancer cells efficiently. A549-WT and A549-ΔCAR cells were infected by replication-selective AdVs at multiplicities of infection ranging from 1 to 300 vpc. Several days later (5 dpi for A549-WT cells, 6 dpi for A549-ΔCAR cells), live cells were stained with crystal violet. Three experiment repeats were performed, representative pictures are shown. (I) Replication-selective variants do not display modified infectivity. A549-WT and A549-ΔCAR cells were infected by 10 vpc of replication-selective AdVs. At 3 hpi, internalized AdV genomes were quantified. n = 4, error bars: maximum-minimum.