ABSTRACT
The presence of anti-thyroid antibodies (ATAs) is a biomarker for the development of thyroid dysfunction induced by anti-programmed cell death-1 antibodies (PD-1-Abs). While patients with thyroid dysfunction reportedly showed better overall survival (OS), it remains unknown if ATAs at baseline can predict OS. Therefore, in this study, we examined the association of ATAs at baseline with OS in non-small cell lung cancer (NSCLC) patients with different levels of programmed cell death-1 ligand 1 (PD-L1) positivity associated with PD-1-Ab treatment efficacy. A total of 81 NSCLC patients treated with PD-1-Abs were evaluated for ATAs at baseline and prospectively for OS. Among the 81 patients, 49 and 32 patients had ≥50% (group A) and <50% (group B) PD-L1 positivity, respectively. Median OS did not differ significantly between patients with (n = 13) and without (n = 36) ATAs at baseline in group A. In contrast, median OS was significantly longer in patients with (n = 10) versus without (n = 22) ATAs at baseline in group B (not reached vs 378 days, respectively; 95% CI, 182 to 574 days, p = 0.049). These findings suggest that the presence of ATAs at baseline is a biomarker to predict better treatment efficacy of PD-1-Abs in NSCLC patients with low PD-L1 positivity, while the difference in OS in those with high PD-L1 positivity may be masked by increased tumor expression of PD-L1.
Key Words: autoimmunity, lung neoplasms, biomarkers, thyroid, programmed cell death-1 (PD-1)
INTRODUCTION
Immune checkpoint inhibitors (ICIs) are promising treatments for several malignancies, including non-small cell lung cancer (NSCLC).1 However, ICIs can cause adverse events, termed immune-related adverse events (irAEs), in various organs including the endocrine glands,2,3 and thyroid dysfunction is one of the most frequent endocrine irAEs in patients treated with anti-programmed cell death-1 (PD-1) antibodies.4-6
Several studies have reported the association of greater anti-tumor effects of ICI treatment with the development of irAEs in cancer patients. For example, skin irAEs induced by nivolumab, an anti-PD-1 antibody, are associated with longer overall survival (OS) in patients with malignant melanoma7 and NSCLC.8 In a previous study, we prospectively analyzed the association of OS with the development of endocrine irAEs and showed that pituitary and thyroid irAEs were associated with longer OS in NSCLC patients.9 Other studies also reported better outcomes in patients who developed thyroid irAEs compared with patients who did not.10,11
While these findings are important for understanding ICI treatment outcomes, it is more critical to predict the therapeutic efficacy of ICIs before initiating treatment. However, only a few clinical parameters, such as age,12 the presence of brain metastases,12,13 and Eastern Cooperative Oncology Group performance status (ECOG PS)12-14 at baseline, are known to be associated with OS in patients with NSCLC. While we previously reported that the presence of anti-thyroid antibodies (ATAs) at baseline is a predictive biomarker for the development of thyroid dysfunction induced by anti-PD-1 antibodies,15-18 a prospective study showed that OS was not associated with the presence of ATAs at baseline in patients with NSCLC treated with anti-PD-1 antibodies.19 However, that study analyzed all patients regardless of background characteristics, which may have affected the outcomes, including programmed cell death-1 ligand 1 (PD-L1) expression.19
In this study, we examined the association of ATAs at baseline with OS in NSCLC patients treated with anti-PD-1 antibodies in terms of the levels of PD-L1 positivity.
METHODS
Patients
Patients with NSCLC who received treatment with nivolumab or pembrolizumab at Nagoya University Hospital from November 2, 2015 to September 30, 2020 were included in the study. Patients who were treated with a combination of pembrolizumab and chemotherapies and patients with more than two malignancies were excluded. All patients were evaluated for ATAs at baseline and prospectively for OS, until death or referral to another hospital. All patients were included in our prospective study analyzing the clinical features of endocrine irAEs (UMIN000019024).
Nivolumab was administered to patients at a dose of either 3 mg/kg or 240 mg every 2 weeks or 480 mg every 4 weeks. Pembrolizumab was administered to patients at a dose of 2 mg/kg or 200 mg every 3 weeks or 400 mg every 6 weeks. Nivolumab or pembrolizumab treatment was continued until disease progression, death, unacceptable severe adverse events, or withdrawal of consent occurred.
Written informed consent was obtained from all patients. This study was performed according to the Declaration of Helsinki and was approved by the Ethical Committee of Nagoya University Hospital.
Assessments
The following baseline factors were evaluated: age, sex, body mass index (BMI), drug use, line of anti-PD-1 antibody treatment, smoking history, tumor histology, ECOG PS, presence of brain, liver, or bone metastases, PD-L1 positivity, and serum ATA levels (anti-thyroglobulin [Tg] antibodies [TgAbs] and anti-thyroid peroxidase [TPO] antibodies [TPOAbs]). TgAb and TPOAb levels were measured using electrochemiluminescence immunoassays (Elecsys Anti-Tg kit and Elecsys Anti-TPO kit, respectively; Roche Diagnostics, Mannheim, Germany). The normal ranges of TgAb and TPOAb were defined as <28 IU/mL and <16 IU/mL, respectively. The relationship between the above baseline factors and OS as a clinical outcome was examined. OS was determined for participants in this study until death from any cause. PD-L1 positivity was determined by testing PD-L1 expression in formalin-fixed tumor tissues using the PD-L1 IHC 22C3 pharmDx assay (Agilent, Santa Clara, CA, USA). The rate of PD-L1 positivity was determined visually by pathologists.
Statistical analyses
Continuous variables of patient characteristics (ie, age, BMI) were expressed as the means ± standard deviations. The differences between continuous variables were tested for significance with the two-sample t-test or Wilcoxon’s rank sum test. Values of nominal variables (ie, sex, drug use, line of anti-PD-1 antibody treatment, tumor histology, ECOG PS, the presence of brain, liver or bone metastases, smoking history, and PD-L1 positivity at baseline) were compared using the χ2 test or Fisher’s exact test. OS was analyzed using the Kaplan-Meier method, expressed as median and 95% confidence interval (CI) and compared using a log-rank test. The end date for the survival analysis was set to September 30, 2021. All statistical tests were two-tailed, and significance was set at a p-value of <0.05. All statistical analyses were performed with IBM SPSS Statistics version 27 (IBM, Chicago, IL, USA).
Patient and public involvement
Patients and the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
RESULTS
Patient characteristics
A total of 143 patients with NSCLC were treated with nivolumab or pembrolizumab at Nagoya University Hospital from November 2, 2015 to September 30, 2020. Among the 143 patients, 23 patients who were treated with a combination of pembrolizumab and chemotherapies and 1 patient who had more than two malignancies were excluded (Fig. 1). Thirty-eight patients whose PD-L1 status was unknown were also excluded from the analysis (Fig. 1). A total of 81 patients were included in this study (Fig. 1). Of the 81 patients enrolled in the study, 69 patients had also been included in our previous studies.9,15,17,20,21
Fig. 1.
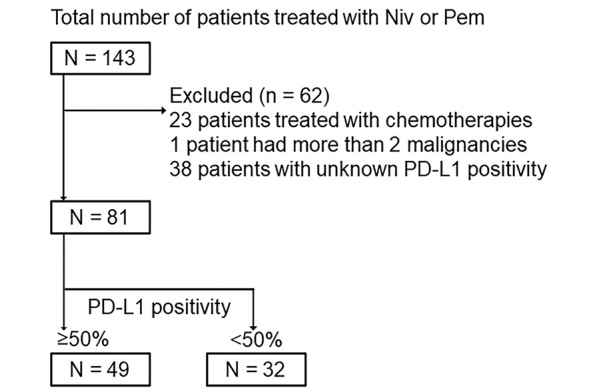
Flow diagram for registration of patients
Niv: nivolumab
Pem: pembrolizumab
PD-L1: programmed cell death-1 ligand 1
The presence of ATAs at baseline was not associated with prolonged OS in NSCLC patients treated with anti-PD-1 antibodies who had ≥50% PD-L1 positivity
Anti-PD-1 antibodies are generally used as first-line treatment in patients who have ≥50% PD-L1 positivity due to better efficacy. Therefore, we examined the prevalence of ≥50% PD-L1 positivity and analyzed the association of OS with the presence of ATAs at baseline according to PD-L1 positivity. Among the 81 patients, 49 and 32 patients had ≥50% and <50% PD-L1 positivity, respectively.
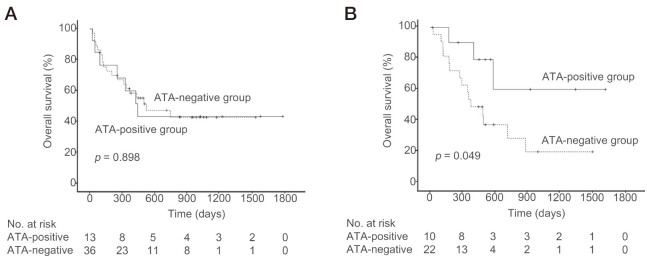
At baseline, among the 49 patients with ≥50% PD-L1 positivity, 13 were positive for TgAbs and/or TPOAbs (ATA-positive group). The remaining 36 patients were negative for ATAs at baseline (ATA-negative group) (Table 1). In the patients who had ≥50% PD-L1 positivity, there was no significant difference in the median OS between the patients who tested positive vs the patients who tested negative for ATAs at baseline (441 days [95% CI 252 to 630 days] vs 521 days [95% CI 106 to 936 days], p = 0.898; Fig. 2A). There were no significant differences in age, BMI, drug use, tumor histology, ECOG PS, presence of brain, liver or bone metastases, or smoking history between the ATA-positive and ATA-negative groups; however, the sex distribution differed significantly between the two groups (Table 1).
Table 1.
Characteristics of patients who had ≥50% programmed cell death-1 ligand-1 positivity
| Characteristics | Total n = 49 |
ATA-negative n = 36 |
ATA-positive n = 13 |
p value |
| Age (years) | 69 ± 10 | 70 ± 8 | 69 ± 11 | 0.781 |
| <70 | 22 | 16 | 6 | 1.000 |
| ≥70 | 27 | 20 | 7 | |
| Sex | ||||
| Female | 13 | 6 | 7 | 0.024 |
| Male | 36 | 30 | 6 | |
| BMI (kg/m2) | 21 ± 3 | 21 ± 3 | 21 ± 4 | 0.451 |
| <25 | 43 | 31 | 12 | 1.000 |
| ≥25 | 6 | 5 | 1 | |
| Drugs | ||||
| Nivolumab | 2 | 1 | 1 | 0.464 |
| Pembrolizumab | 47 | 35 | 12 | |
| Tumor histology | ||||
| SCC | 12 | 8 | 4 | 0.708 |
| Non-SCC | 37 | 28 | 9 | |
| ECOG PS | ||||
| <2 | 46 | 34 | 12 | 1.000 |
| ≥2 | 3 | 2 | 1 | |
| Brain metastases | ||||
| Yes | 5 | 5 | 0 | 0.306 |
| No | 44 | 31 | 13 | |
| Liver metastases | ||||
| Yes | 7 | 4 | 3 | 0.363 |
| No | 42 | 32 | 10 | |
| Bone metastases | ||||
| Yes | 14 | 12 | 2 | 0.297 |
| No | 35 | 24 | 11 | |
| Smoking history | ||||
| Current/former | 39 | 30 | 9 | 0.422 |
| Never | 10 | 6 | 4 |
Data are represented by the mean ± SD or n.
ATA: anti-thyroid antibody
BMI: body mass index
SCC: squamous cell carcinoma
Non-SCC: non-squamous cell carcinoma
ECOG PS: Eastern Cooperative Oncology Group performance status
Fig. 2.
Overall survival (OS) in patients with and without anti-thyroid antibodies (ATAs) at baseline
Solid and dashed lines indicate the OS of patients who did and did not have ATAs at baseline, respectively.
Fig. 2A: OS in patients with ≥50% PD-L1 positivity.
Fig. 2B: OS in patients with <50% PD-L1 positivity.
The presence of ATAs at baseline was associated with prolonged OS in NSCLC patients treated with anti-PD-1 antibodies who had <50% PD-L1 positivity
Among the 32 patients with <50% PD-L1 positivity at baseline, 10 were positive for ATAs (Table 2). The remaining 22 patients were negative for ATAs at baseline. In the patients who had <50% PD-L1 positivity, the median OS was significantly longer for the patients who tested positive for ATAs at baseline than for those who tested negative (not reached vs 378 days [95% CI 182 to 574 days], p = 0.049; Fig. 2B). There were no significant differences in age, BMI, drug use, tumor histology, ECOG PS, presence of brain or liver metastases, or smoking history between the ATA-positive and ATA-negative groups; however, the presence of bone metastasis and prevalence of PD-L1 positivity differed significantly between the two groups (Table 2).
Table 2.
Characteristics of patients who had <50% programmed cell death-1 ligand-1 positivity
| Characteristics | Total n = 32 |
ATA-negative n = 22 |
ATA-positive n = 10 |
p value |
| Age (years) | 68 ± 10 | 73 ± 6 | 62 ± 11 | 0.163 |
| <70 | 14 | 10 | 4 | 1.000 |
| ≥70 | 18 | 12 | 6 | |
| Sex | ||||
| Female | 7 | 5 | 2 | 1.000 |
| Male | 25 | 17 | 8 | |
| BMI (kg/m2) | 22 ± 3 | 21 ± 4 | 22 ± 2 | 0.703 |
| <25 | 26 | 18 | 8 | 1.000 |
| ≥25 | 6 | 4 | 2 | |
| Drugs | ||||
| Nivolumab | 18 | 15 | 3 | 0.062 |
| Pembrolizumab | 14 | 7 | 7 | |
| Tumor histology | ||||
| SCC | 13 | 8 | 5 | 0.699 |
| Non-SCC | 19 | 14 | 5 | |
| ECOG PS | ||||
| <2 | 30 | 20 | 10 | 1.000 |
| ≥2 | 2 | 2 | 0 | |
| Brain metastases | ||||
| Yes | 6 | 4 | 2 | 1.000 |
| No | 26 | 18 | 8 | |
| Liver metastases | ||||
| Yes | 4 | 4 | 0 | 0.283 |
| No | 28 | 18 | 10 | |
| Bone metastases | ||||
| Yes | 8 | 8 | 0 | 0.035 |
| No | 24 | 14 | 10 | |
| Smoking history | ||||
| Current/former | 26 | 18 | 8 | 1.000 |
| Never | 6 | 4 | 2 | |
| PD-L1 positivity | ||||
| <1% | 8 | 8 | 0 | 0.035 |
| 1–49% | 24 | 14 | 10 |
Data are represented by the mean ± SD or n.
ATA: anti-thyroid antibody
BMI: body mass index
SCC: squamous cell carcinoma
Non-SCC: non-squamous cell carcinoma
ECOG PS: Eastern Cooperative Oncology Group performance status
PD-L1: programmed cell death-1 ligand 1
The association of PD-L1 positivity with OS
When the association of PD-L1 positivity with OS was analyzed in this cohort, median OS did not differ significantly between patients who had ≥50% PD-L1 positivity (n = 49) and those who had <50% PD-L1 positivity (n = 32) (499 days [95% CI 144 to 853 days] vs 493 days [95% CI 248 to 738 days], p = 0.964).
DISCUSSION
This real-world prospective study revealed that positive ATAs at baseline are associated with longer OS in NSCLC patients treated with anti-PD-1 antibodies only if the patients have <50% PD-L1 positivity.
While ICI treatment showed anti-tumor effects in patients with advanced malignancies, including NSCLC, these effects were observed only in a limited subset of patients.1 Therefore, it is important to identify patients who will show better responses before initiating ICI treatment. Several studies have shown that the development of irAEs induced by ICIs is associated with better outcomes.22 As for endocrine irAEs, the development of thyroid dysfunction induced by anti-PD-1 antibodies was associated with prolonged OS in NSCLC patients in prospective9,10 and retrospective studies.23,24 However, the development of irAEs cannot be used as a biomarker to predict treatment outcomes before the initiation of ICIs. Here, we showed that the presence of ATAs at baseline was a biomarker for better OS in patients with <50% PD-L1 positivity who received anti-PD-1 antibodies. In previous studies, we clarified that patients who tested positive for ATAs at baseline developed thyroid dysfunction at a higher frequency than those who tested negative.15-18 Thus, to the best of our knowledge, we have demonstrated for the first time that a serological biomarker for an irAE also has the potential to be a biomarker for OS.
Several studies have examined the association of ATAs with treatment outcomes. Toi et al reported that the prevalence of ATAs at baseline was significantly higher in responder patients with NSCLC (both complete response and partial response) than non-responder patients (stable disease and progressive disease). In a retrospective study of 70 NSCLC patients treated with nivolumab,25 9 of 21 responder patients (43%) vs 6 of 49 non-responder patients (12%) had ATAs at baseline; OS was not examined in their study. Subsequently, the same group reported that ATAs at baseline were not associated with OS or progression-free survival (PFS) in another retrospective analysis of a larger number of NSCLC patients (n = 137), in which all patients were analyzed regardless of treatment lines and PD-L1 positivity.19 Another study also found that ATA status at baseline was not associated with OS or PFS in patients treated with anti-PD-1 antibodies; the study included not only patients with NSCLC but also those with other malignancies (renal cell carcinoma and melanoma).26 In this study, we analyzed the association of ATAs at baseline with OS in NSCLC patients treated with anti-PD-1 antibodies in terms of ≥50% or <50% PD-L1 positivity and showed that the presence of ATAs at baseline was significantly associated with better OS in patients with <50% PD-L1 positivity.
In a phase 3 study comparing pembrolizumab with platinum-based chemotherapy as the first-line treatment in NSCLC patients with ≥50% PD-L1 positivity, OS was significantly prolonged in the pembrolizumab group.27,28 In addition, the hazard ratio for OS was the lowest in patients with ≥50% PD-L1 positivity among the analyses of patients with ≥50%, ≥20% and ≥1% PD-L1 positivity, suggesting an association of higher tumor PD-L1 positivity with greater efficacy of pembrolizumab. Based on these clinical findings, pembrolizumab is preferentially used as the first-line treatment for NSCLC patients who have high PD-L1 positivity (≥50%). This may explain why the association of OS with the presence of ATAs at baseline was different between patients with ≥50% and those with <50% PD-L1 positivity in this study. Although median OS did not differ between patients with ≥50% PD-L1 positivity and patients with < 50% PD-L1 positivity, this real-world study included not only patients treated with pembrolizumab, but also patients treated with nivolumab. Since a clinical trial of nivolumab showed that a higher cutoff value of PD-L1 expression (≥1, ≥5 and ≥10%) was associated with a stronger effect on OS prolongation29, the cutoff value for PD-L1 positivity that determines therapeutic efficacy may vary among drugs.
Possible explanations for the association of ATAs with better OS in patients who have <50% PD-L1 positivity are as follows: 1) common epitopes exist between thyroid autoantigens and cancer antigens, 2) common human leukocyte antigen (HLA) types restrict the T-cell response to both thyroid antigens and cancer antigens, and 3) the better response of anti-PD-1 antibodies in NSCLC patients with ≥ 50% PD-L1 positivity may be masked by increased tumor expression of PD-L1. Thus, PD-L1 expression in tumor cells is upregulated by interferon-γ produced by infiltrated T cells,30 and high PD-L1 positivity in tumors may reflect greater T-cell infiltration. In this case, greater efficacy of anti-PD-1 antibodies would be expected. If higher expression of tumor PD-L1 induces greater efficacy of anti-PD-1 antibodies, the difference in OS, if any, between ATA-positive and ATA-negative groups could be subtle in patients with ≥50% PD-L1 positivity.
BMI, ECOG PS, age and genetic variations are reported to be possible markers for better treatment outcomes with ICIs. BMI at baseline was reported as a possible marker affecting OS, although there was no difference in BMI between ATA-positive and ATA-negative groups in this study. In previous studies, the mean BMI was higher in patients who developed overt thyroid dysfunction induced by blockade of PD-1 or PD-L1 than in those who did not (27.3 ± 6.0 vs 24.9 ± 4.5),31 and obesity was also associated with better efficacy of PD-1/PD-L1 blockade in cancer patients.32 This suggests that measuring BMI at baseline may be useful to predict not only a risk of thyroid irAEs but also the anti-tumor efficacy of ICIs. ECOG PS and age were also reported to be associated with better OS. Using multivariate analysis, several studies also reported that ECOG PS <2 was significantly associated with better OS in NSCLC patients.12-14 In contrast, other investigators reported that age ≥7012,13 or age ≤7514 are significant factors associated with better OS in NSCLC patients, indicating that the association of age with better OS remains inconclusive. It was also reported that a polygenic risk score, determined based on genetic variations associated with thyroid autoimmunity, was high in patients who developed atezolizumab-induced thyroid dysfunction and that patients with a high polygenic risk score were at a lower risk of death from breast cancer,33 suggesting the usefulness of genetic analysis at baseline. Taken together, it may be useful to consider the above parameters as well as ATA status at baseline for the prediction of treatment efficacy in PD-1 blockade.
In the analysis of 49 patients who had ≥50% PD-L1 positivity, the sex distribution differed significantly between the ATA-positive and ATA-negative groups. The sex distribution was reasonable, because females have thyroid autoimmunity more frequently than males.34,35 In the 32 patients who had <50% PD-L1 positivity, there was a significant difference in the prevalence of patients with <1% PD-L1 positivity between the ATA-positive and ATA-negative groups. This difference in background characteristics may have affected the OS results in this study. Since the reported cutoff values of PD-L1 positivity to identify patients showing better outcomes differed between pembrolizumab and nivolumab, future studies in a larger number of NSCLC patients treated with each drug are needed to confirm the association of positive ATAs at baseline with longer OS in patients with <50% PD-L1 positivity.
We found a significant difference in the frequency of bone metastasis between the ATA-positive and ATA-negative groups among patients with <50% PD-L1 positivity. Patients with advanced malignancies sometimes develop metastatic lesions and often have a poor prognosis. Regarding bone metastasis, an association between bone metastasis and poor OS in NSCLC patients treated with ICI monotherapy was found in some reports13,36-38 but not others.39-41 Therefore, it remains inconclusive if the presence of bone metastasis had an effect on OS in this study.
This study had some limitations. First, the numbers of patients analyzed in this study were relatively small. Second, the lower prevalence of <1% PD-L1 positivity in ATA-positive versus ATA-negative groups may have affected OS in the analysis of patients who had <50% PD-L1 positivity. Third, this study included patients who had different treatment histories before or after anti-PD-1 antibody treatment, which may have affected OS.
CONCLUSIONS
Our data showed the association of positive ATAs at baseline with longer OS in the patients who had <50% PD-L1 positivity, but not in those with ≥50% PD-L1 positivity. These results indicate that the presence of ATAs at baseline may predict the treatment outcomes in NSCLC patients with <50% PD-L1 positivity receiving treatment with anti-PD-1 antibodies. Therefore, the presence of ATAs at baseline is a clinically relevant biomarker for ICI treatment outcomes.
SOURCES OF FUNDING
This research received no specific grant from any funding agencies in the public, commercial or not-for-profit sectors.
CONFLICTS OF INTEREST
SI received personal fees from Ono Pharmaceutical Company, Bristol-Myers Squibb, and MSD KK outside of this study. MA received a grant from Kyowa Kirin Co Ltd, outside of this study. THas received personal fees from Ono Pharmaceutical Company, Bristol-Myers Squibb, and MSD KK. TOn received personal fees from MSD KK outside of this study. YA received grants and personal fees from Ono Pharmaceutical Company, and personal fees from Bristol Myers Squibb and MSD KK outside of this study. HA received grants from Ono Pharmaceutical Company and MSD KK and personal fees from Ono Pharmaceutical Company, Bristol Myers Squibb, and MSD KK outside of this study. The remaining authors have nothing to disclose.
Editorial Note
This article has been selected as an “Editors’ Choice” by the editorial board.
Abbreviations
- ATAs
anti-thyroid antibodies
- CI
confidence interval
- ECOG PS
Eastern Cooperative Oncology Group performance status
- ICIs
immune checkpoint inhibitors
- irAEs
immune-related adverse events
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PD-L1
programmed cell death-1 ligand 1
- PD-1
programmed cell death-1
REFERENCES
- 1.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6(1):8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed]
- 2.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed]
- 3.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed]
- 4.Arima H, Iwama S, Inaba H, et al. Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: clinical guidelines of the Japan Endocrine Society. Endocr J. 2019;66(7):581–586. doi: 10.1507/endocrj.EJ19-0163. [DOI] [PubMed]
- 5.Iwama S, Kobayashi T, Arima H. Clinical Characteristics, Management, and Potential Biomarkers of Endocrine Dysfunction Induced by Immune Checkpoint Inhibitors. Endocrinol Metab (Seoul). 2021;36(2):312–321. doi: 10.3803/EnM.2021.1007. [DOI] [PMC free article] [PubMed]
- 6.Zhai Y, Ye X, Hu F, et al. Endocrine toxicity of immune checkpoint inhibitors: a real-world study leveraging US Food and Drug Administration adverse events reporting system. J Immunother Cancer. 2019;7(1):286. doi: 10.1186/s40425-019-0754-2. [DOI] [PMC free article] [PubMed]
- 7.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res. 2016;22(4):886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed]
- 8.Haratani K, Hayashi H, Chiba Y, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018;4(3):374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed]
- 9.Kobayashi T, Iwama S, Yasuda Y, et al. Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both malignant melanoma and non-small cell lung carcinoma: a prospective study. J Immunother Cancer. 2020;8(2):e000779. doi: 10.1136/jitc-2020-000779. [DOI] [PMC free article] [PubMed]
- 10.Osorio JC, Ni A, Chaft JE, et al. Antibody-Mediated Thyroid Dysfunction During T-cell Checkpoint Blockade in Patients with Non-Small Cell Lung Cancer. Ann Oncol. 2017;28(3):583–589. doi: 10.1093/annonc/mdw640. [DOI] [PMC free article] [PubMed]
- 11.Kim HI, Kim M, Lee SH, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology. 2017;7(1):e1375642. doi: 10.1080/2162402X.2017.1375642. [DOI] [PMC free article] [PubMed]
- 12.Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479–485. doi: 10.1007/s00432-018-2805-3. [DOI] [PubMed]
- 13.Baldini E, Lunghi A, Cortesi E, et al. Immune-related adverse events correlate with clinical outcomes in NSCLC patients treated with nivolumab: The Italian NSCLC expanded access program. Lung Cancer. 2020;140:59–64. doi: 10.1016/j.lungcan.2019.12.014. [DOI] [PubMed]
- 14.Naqash AR, Ricciuti B, Owen DH, et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother. 2020;69(7):1177–1187. doi: 10.1007/s00262-020-02536-5. [DOI] [PMC free article] [PubMed]
- 15.Kobayashi T, Iwama S, Yasuda Y, et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J Endocr Soc. 2018;2(3):241–251. doi: 10.1210/js.2017-00432. [DOI] [PMC free article] [PubMed]
- 16.Kimbara S, Fujiwara Y, Iwama S, et al. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci. 2018;109(11):3583–3590. doi: 10.1111/cas.13800. [DOI] [PMC free article] [PubMed]
- 17.Okada N, Iwama S, Okuji T, et al. Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer. 2020;122(6):771–777. doi: 10.1038/s41416-020-0736-7. [DOI] [PMC free article] [PubMed]
- 18.Iwama S, Kobayashi T, Yasuda Y, et al. Increased risk of thyroid dysfunction by PD-1 and CTLA-4 blockade in patients without thyroid autoantibodies at baseline. J Clin Endocrinol Metab. 2022;107(4):e1620-e1630. doi: 10.1210/clinem/dgab829. [DOI] [PubMed]
- 19.Toi Y, Sugawara S, Sugisaka J, et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2019;5(3):376–383. doi: 10.1001/jamaoncol.2018.5860. [DOI] [PMC free article] [PubMed]
- 20.Kobayashi T, Iwama S, Sugiyama D, et al. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J Immunother Cancer. 2021;9(5):e002493. doi: 10.1136/jitc-2021-002493. [DOI] [PMC free article] [PubMed]
- 21.Yasuda Y, Iwama S, Sugiyama D, et al. CD4(+) T cells are essential for the development of destructive thyroiditis induced by anti-PD-1 antibody in thyroglobulin-immunized mice. Sci Transl Med. 2021;13(593):eabb7495. doi: 10.1126/scitranslmed.abb7495. [DOI] [PubMed]
- 22.Petrelli F, Grizzi G, Ghidini M, et al. Immune-related Adverse Events and Survival in Solid Tumors Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J Immunother. 2020;43(1):1–7. doi: 10.1097/CJI.0000000000000300. [DOI] [PubMed]
- 23.Yamauchi I, Yasoda A, Matsumoto S, et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One. 2019;14(5):e0216954. doi: 10.1371/journal.pone.0216954. [DOI] [PMC free article] [PubMed]
- 24.Grangeon M, Tomasini P, Chaleat S, et al. Association Between Immune-related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Non-small-cell Lung Cancer. Clin Lung Cancer. 2019;20(3):201–207. doi: 10.1016/j.cllc.2018.10.002. [DOI] [PubMed]
- 25.Toi Y, Sugawara S, Kawashima Y, et al. Association of Immune-Related Adverse Events with Clinical Benefit in Patients with Advanced Non-Small-Cell Lung Cancer Treated with Nivolumab. Oncologist. 2018;23(11):1358–1365. doi: 10.1634/theoncologist.2017-0384. [DOI] [PMC free article] [PubMed]
- 26.Basak EA, van der Meer JWM, Hurkmans DP, et al. Overt Thyroid Dysfunction and Anti-Thyroid Antibodies Predict Response to Anti-PD-1 Immunotherapy in Cancer Patients. Thyroid. 2020;30(7):966–973. doi: 10.1089/thy.2019.0726. [DOI] [PubMed]
- 27.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019;37(7):537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed]
- 28.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed]
- 29.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed]
- 30.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2020;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed]
- 31.Pollack R, Ashash A, Cahn A, Rottenberg Y, Stern H, Dresner-Pollak R. Immune Checkpoint Inhibitor-induced Thyroid Dysfunction Is Associated with Higher Body Mass Index. J Clin Endocrinol Metab. 2020;105(10):dgaa458. doi: 10.1210/clinem/dgaa458. [DOI] [PubMed]
- 32.Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25(1):141–151. doi: 10.1038/s41591-018-0221-5. [DOI] [PMC free article] [PubMed]
- 33.Khan Z, Hammer C, Carroll J, et al. Genetic variation associated with thyroid autoimmunity shapes the systemic immune response to PD-1 checkpoint blockade. Nat Commun. 2021;12(1):3355. doi: 10.1038/s41467-021-23661-4. [DOI] [PMC free article] [PubMed]
- 34.Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13(4–5):391–397. doi: 10.1016/j.autrev.2014.01.007. [DOI] [PubMed]
- 35.Merrill SJ, Mu Y. Thyroid autoimmunity as a window to autoimmunity: An explanation for sex differences in the prevalence of thyroid autoimmunity. J Theor Biol. 2015;375:95–100. doi: 10.1016/j.jtbi.2014.12.015. [DOI] [PubMed]
- 36.Li X, Wang L, Chen S, et al. Adverse impact of bone metastases on clinical outcomes of patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer. 2020;11(10):2812–2819. doi: 10.1111/1759-7714.13597. [DOI] [PMC free article] [PubMed]
- 37.Qin A, Zhao S, Miah A, et al. Bone metastases, skeletal-related events, and survival in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. J Natl Compr Canc Netw. 2021;19(8):915–921. doi: 10.6004/jnccn.2020.7668. [DOI] [PMC free article] [PubMed]
- 38.Yoneda T, Sone T, Koba H, et al. Long-term survival of patients with non-small cell lung cancer treated with immune checkpoint inhibitor monotherapy in real-world settings. Clin Lung Cancer. 2022;23(6):467–476. doi: 10.1016/j.cllc.2022.03.008. [DOI] [PubMed]
- 39.Garde-Noguera J, Martin-Martorell P, De Julián M, et al. Predictive and prognostic clinical and pathological factors of nivolumab efficacy in non-small-cell lung cancer patients. Clin Transl Oncol. 2018;20(8):1072–1079. doi: 10.1007/s12094-017-1829-5. [DOI] [PubMed]
- 40.Qiao M, Zhou F, Hou L, et al. Efficacy of immune-checkpoint inhibitors in advanced non-small cell lung cancer patients with different metastases. Ann Transl Med. 2021;9(1):34. doi: 10.21037/atm-20-1471. [DOI] [PMC free article] [PubMed]
- 41.Deng J, Gao M, Gou Q, et al. Organ-specific efficacy in advanced non-small cell lung cancer patients treated with first-line single-agent immune checkpoint inhibitors. Chin Med J (Engl). 2022;135(12):1404–1413. doi: 10.1097/CM9.0000000000002217. [DOI] [PMC free article] [PubMed]