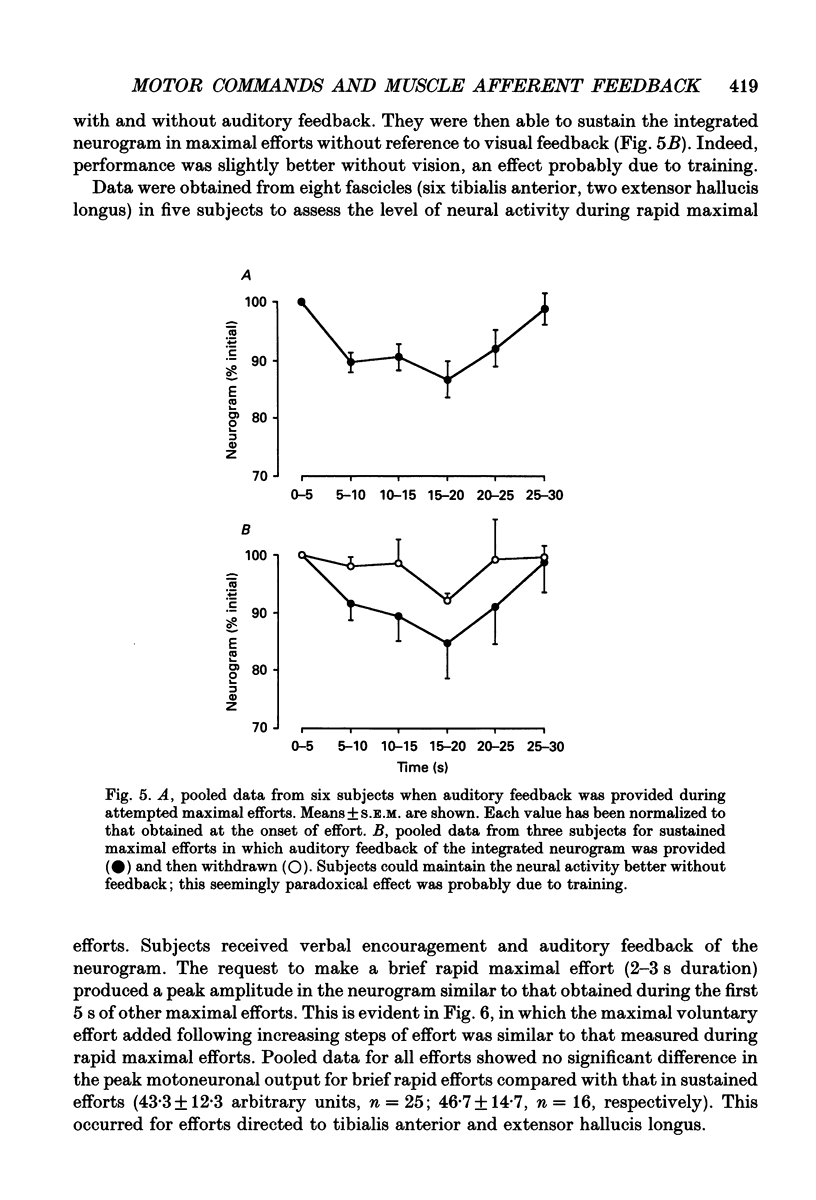
Abstract
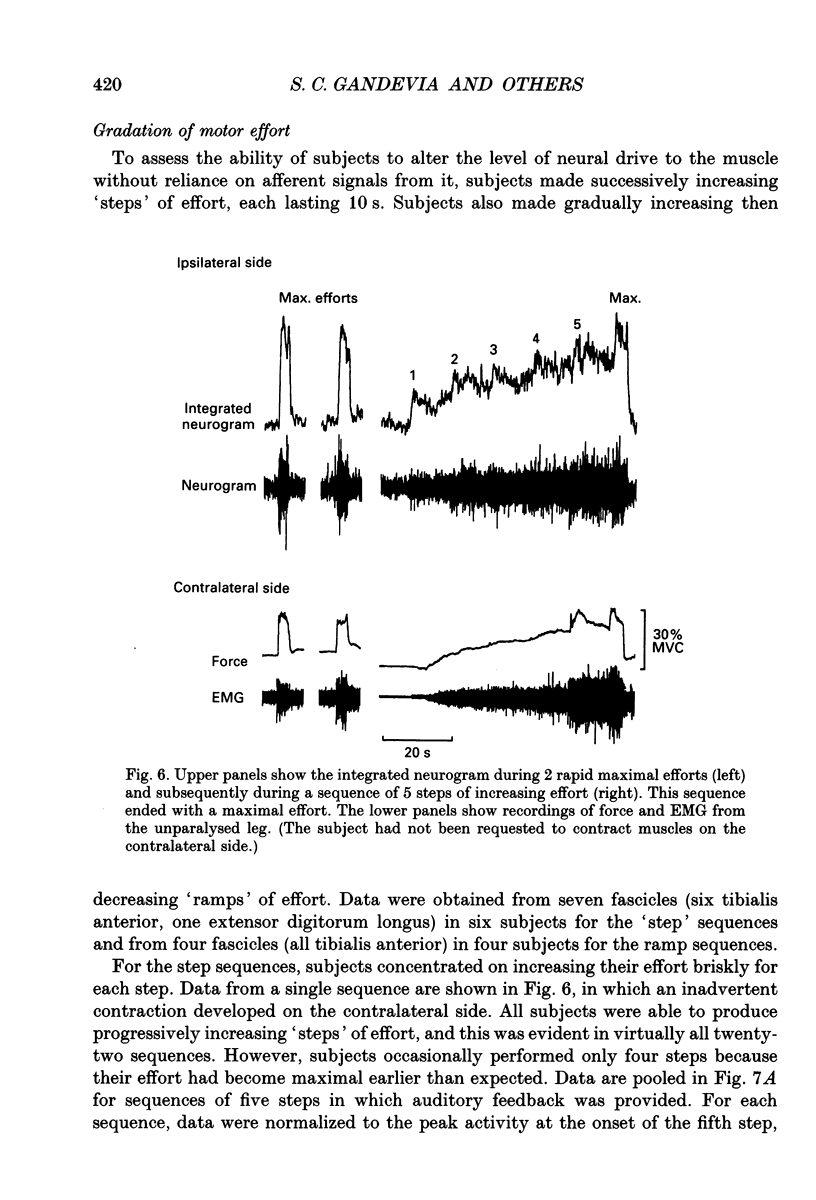
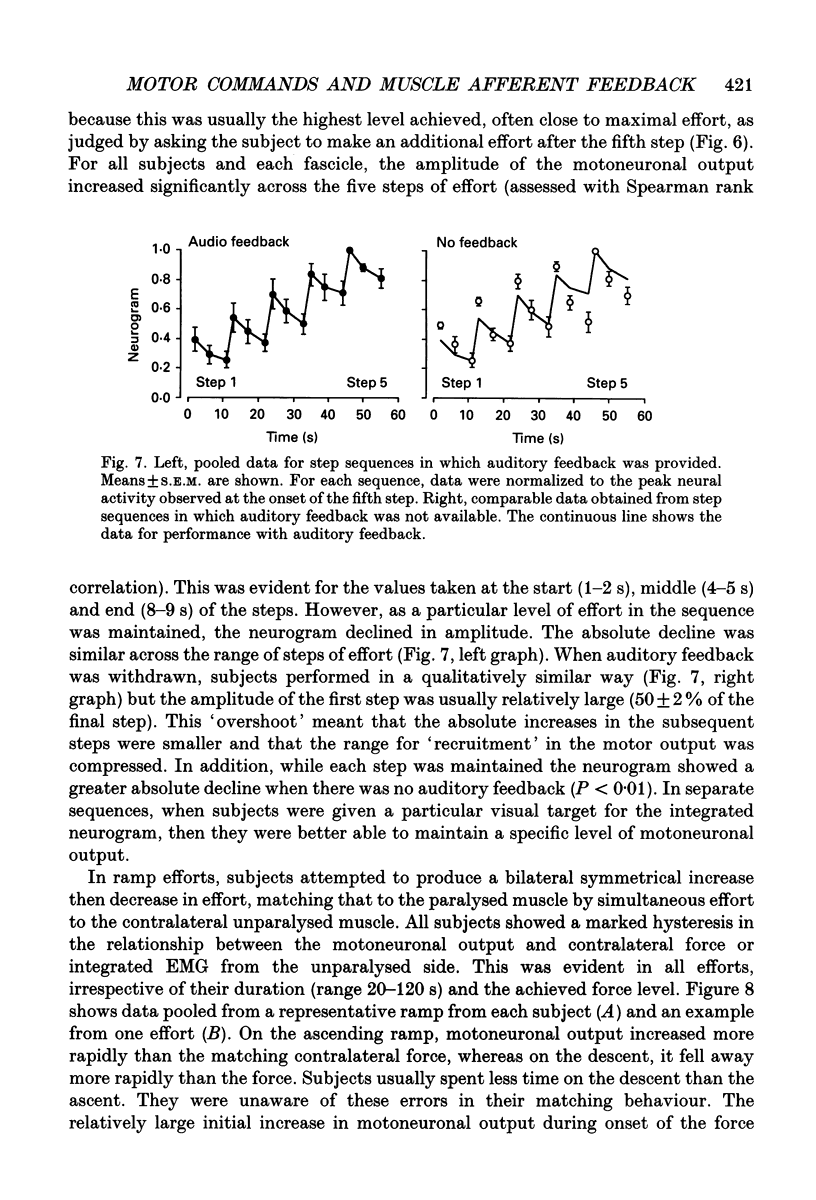
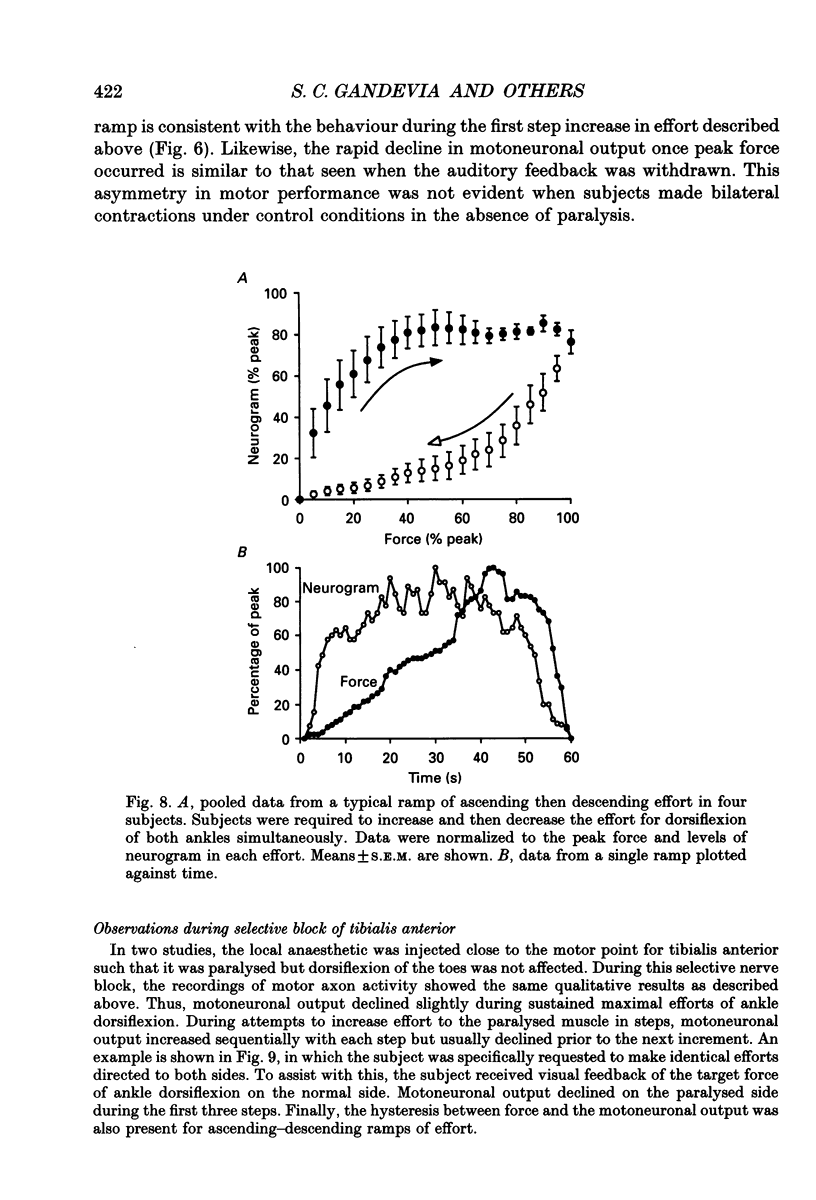
1. The study was designed to determine the degree to which normal subjects can control motoneurones innervating a leg muscle when acutely deprived of muscle afferent feedback. Microneurographic recordings were made from eighteen motor fascicles in the common peroneal nerve, of which thirteen innervated tibialis anterior and five toe dorsiflexor muscles. The nerve was then blocked completely at a distal site near the fibular head with local anaesthetic. A sequence of tests was performed with each fascicle to determine the degree to which the subject could control the motoneuronal drive to the paralysed muscle. 2. During a complete distal block of the common peroneal nerve, motoneurones innervating tibialis anterior were frequently activated during weak attempted contraction of the synergist toe extensors and vice versa. 3. When subjects attempted contractions of the paralysed muscles at a constant effort, pressure applied to the dorsum of the foot caused relatively small changes in the level of neural output, producing a small increase in motoneuronal drive to tibialis anterior, but no consistent change in the drive to toe extensor fascicles. 4. Subjects were able to increase the motoneuronal drive to the paralysed tibialis anterior in five steps of effort each lasting 10 s. The level of motor output increased linearly with step number, but declined as the step was maintained, more so when auditory feedback was withdrawn. 5. There was hysteresis in the relationship between motoneuronal output and force (measured on the contralateral side) during attempts to make slowly increasing then decreasing ramps of effort on both sides over 20-120 s. Motor drive to the paralysed muscle increased disproportionately rapidly compared with contralateral force when subjects attempted bilaterally symmetrical increasing efforts. 6. Subjects attempted to activate the paralysed muscle group maximally for 20-30 s with auditory feedback of the neurogram and verbal encouragement. There was a small statistically significant reduction in the motoneuronal output 5-10 s into the 30 s effort but, with further encouragement, it recovered towards the end of the effort. 7. When compared directly in the same recording sequences, attempts to make rapid brief maximal efforts (2-3 s duration) produced the same motoneuronal output as attempts to make sustained efforts. 8. Similar results occurred when the motoneuronal output to tibialis anterior was recorded during a selective distal block of tibialis anterior sparing toe dorsiflexors.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


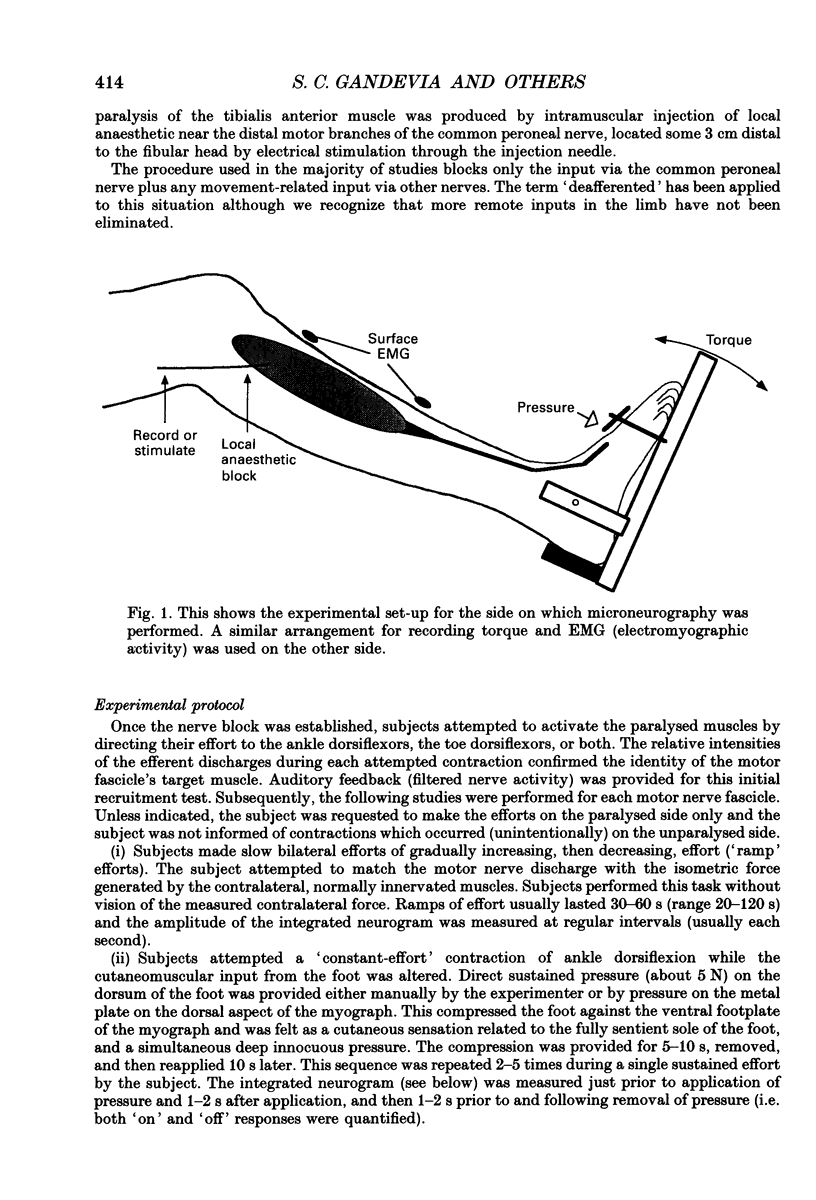

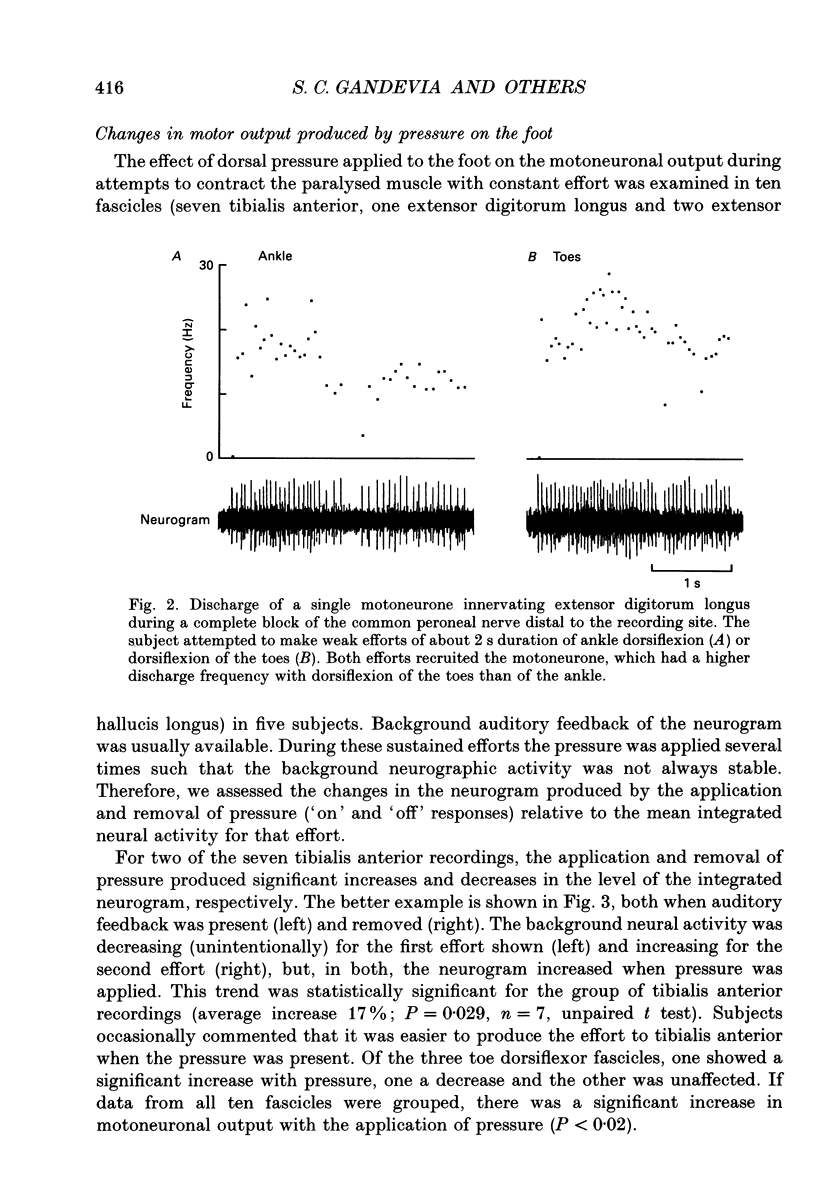
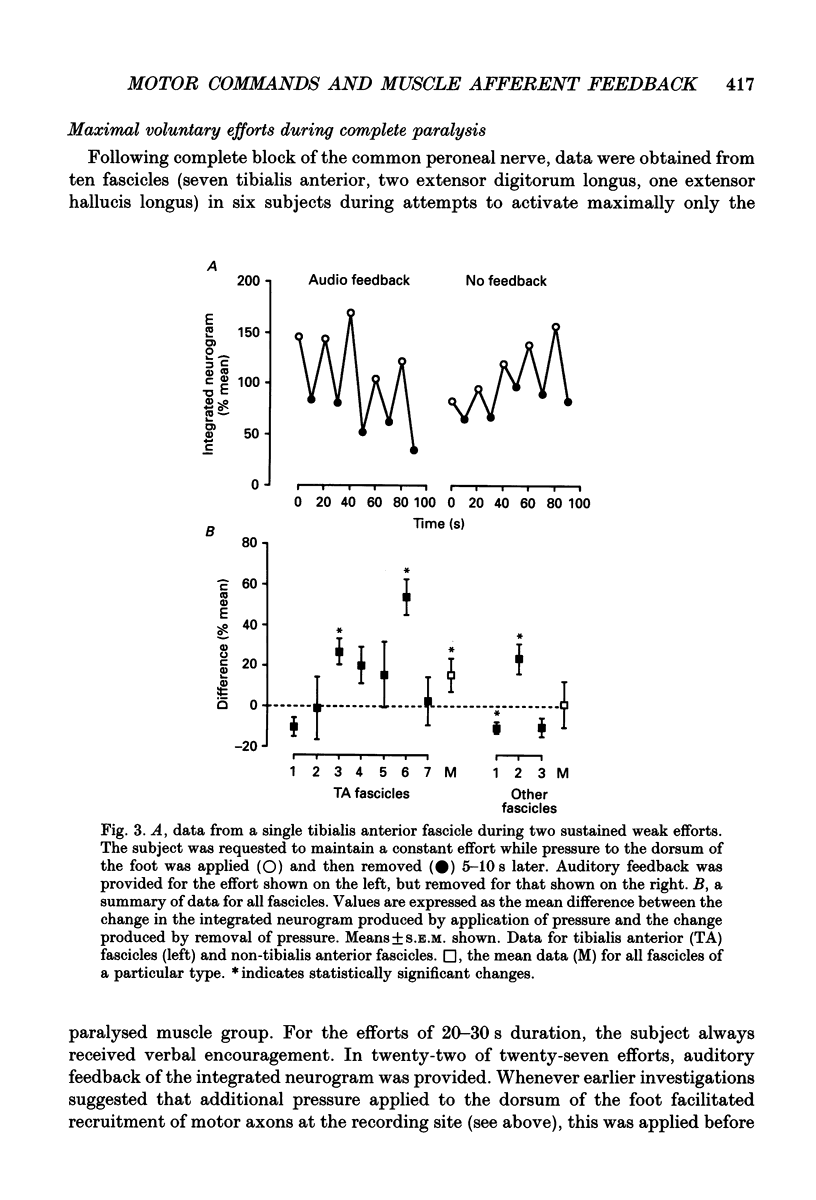
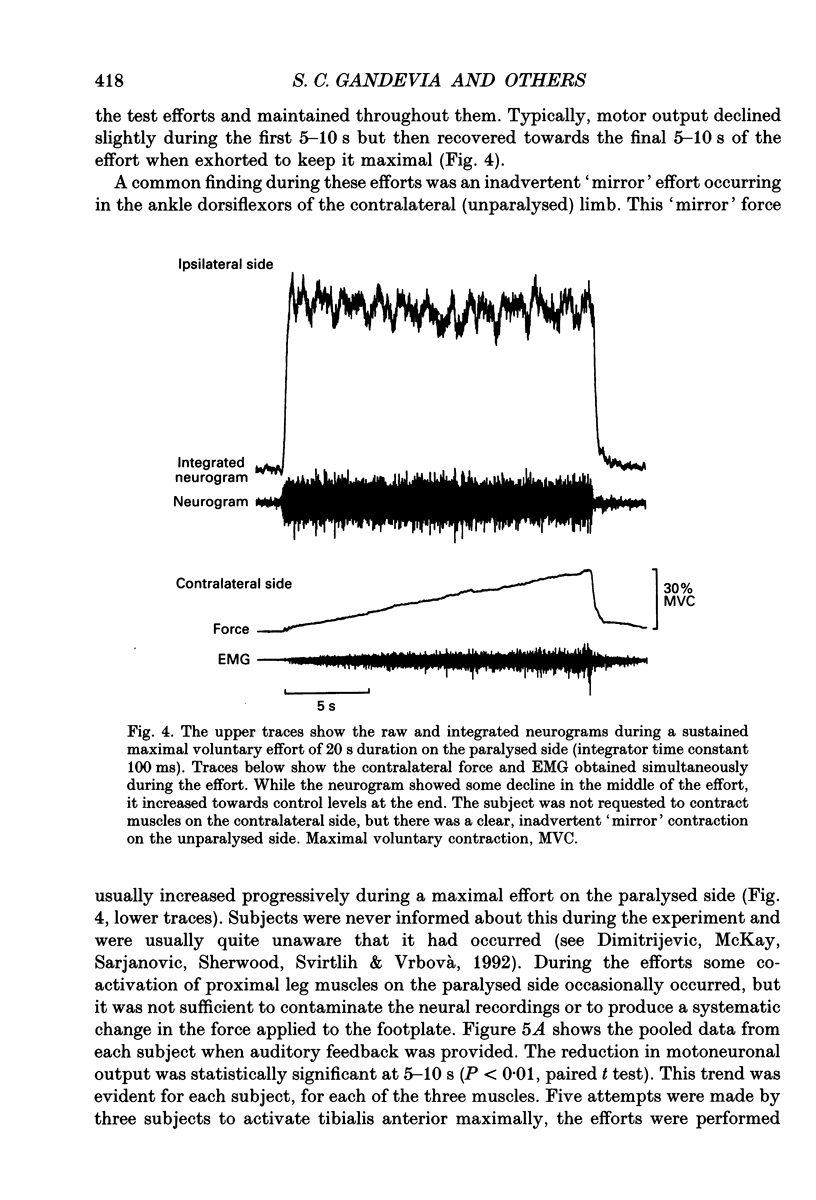
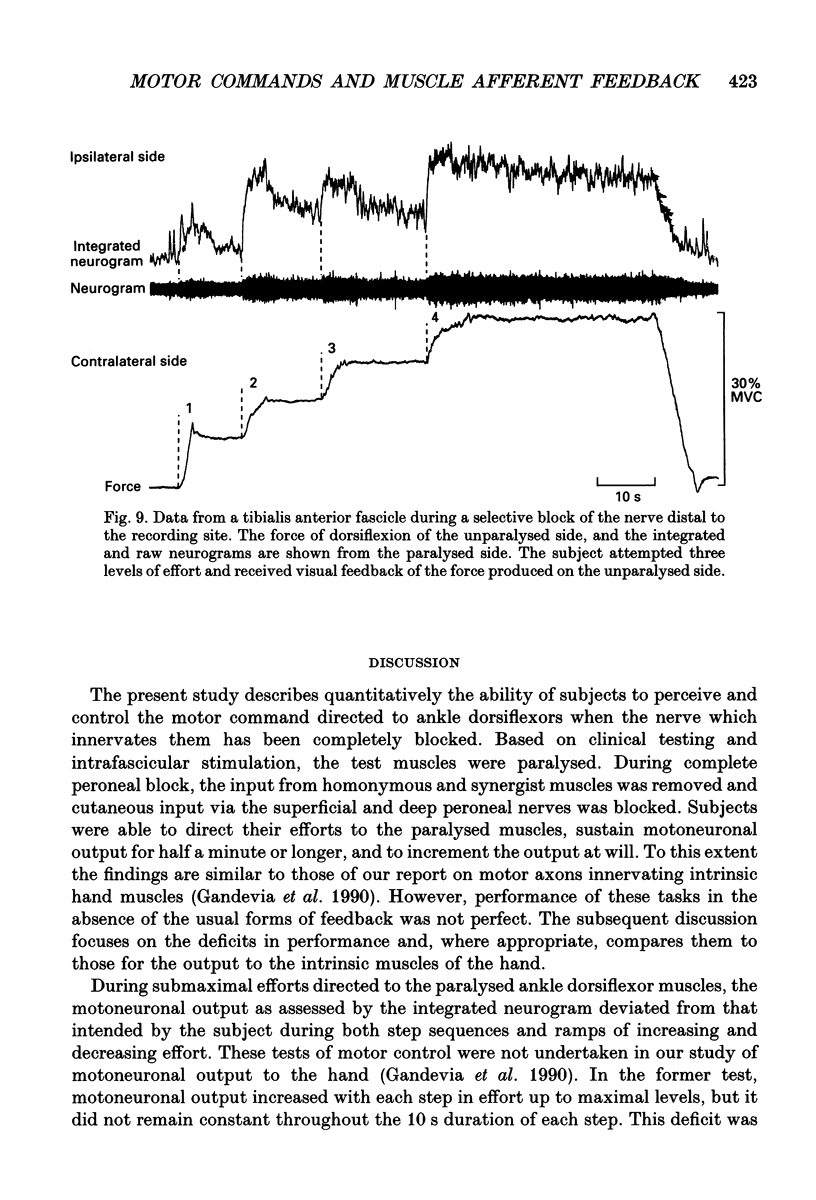




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aniss A. M., Gandevia S. C., Burke D. Reflex responses in active muscles elicited by stimulation of low-threshold afferents from the human foot. J Neurophysiol. 1992 May;67(5):1375–1384. doi: 10.1152/jn.1992.67.5.1375. [DOI] [PubMed] [Google Scholar]
- Aniss A. M., Gandevia S. C., Milne R. J. Changes in perceived heaviness and motor commands produced by cutaneous reflexes in man. J Physiol. 1988 Mar;397:113–126. doi: 10.1113/jphysiol.1988.sp016991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIGLAND B., LIPPOLD O. C. The relation between force, velocity and integrated electrical activity in human muscles. J Physiol. 1954 Jan;123(1):214–224. doi: 10.1113/jphysiol.1954.sp005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic M. R., McKay W. B., Sarjanovic I., Sherwood A. M., Svirtlih L., Vrbovà G. Co-activation of ipsi- and contralateral muscle groups during contraction of ankle dorsiflexors. J Neurol Sci. 1992 May;109(1):49–55. doi: 10.1016/0022-510x(92)90092-y. [DOI] [PubMed] [Google Scholar]
- Forget R., Lamarre Y. Rapid elbow flexion in the absence of proprioceptive and cutaneous feedback. Hum Neurobiol. 1987;6(1):27–37. [PubMed] [Google Scholar]
- Gandevia S. C., Macefield G., Burke D., McKenzie D. K. Voluntary activation of human motor axons in the absence of muscle afferent feedback. The control of the deafferented hand. Brain. 1990 Oct;113(Pt 5):1563–1581. doi: 10.1093/brain/113.5.1563. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C., McCloskey D. I. Interpretation of perceived motor commands by reference to afferent signals. J Physiol. 1978 Oct;283:493–499. [PMC free article] [PubMed] [Google Scholar]
- Gandevia S. C., McCloskey D. I. Sensations of heaviness. Brain. 1977 Jun;100(2):345–354. doi: 10.1093/brain/100.2.345. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C., Rothwell J. C. Knowledge of motor commands and the recruitment of human motoneurons. Brain. 1987 Oct;110(Pt 5):1117–1130. doi: 10.1093/brain/110.5.1117. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C. The perception of motor commands or effort during muscular paralysis. Brain. 1982 Mar;105(Pt 1):151–159. doi: 10.1093/brain/105.1.151. [DOI] [PubMed] [Google Scholar]
- Garnett R., Stephens J. A. The reflex responses of single motor units in human first dorsal interosseous muscle following cutaneous afferent stimulation. J Physiol. 1980 Jun;303:351–364. doi: 10.1113/jphysiol.1980.sp013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. M., McCloskey D. I., Matthews P. B. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95(4):705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Hannerz J. Discharge properties of motor units in relation to recruitment order in voluntary contraction. Acta Physiol Scand. 1974 Jul;91(3):374–385. doi: 10.1111/j.1748-1716.1974.tb05692.x. [DOI] [PubMed] [Google Scholar]
- Hoffer J. A., Sugano N., Loeb G. E., Marks W. B., O'Donovan M. J., Pratt C. A. Cat hindlimb motoneurons during locomotion. II. Normal activity patterns. J Neurophysiol. 1987 Feb;57(2):530–553. doi: 10.1152/jn.1987.57.2.530. [DOI] [PubMed] [Google Scholar]
- Macefield G., Hagbarth K. E., Gorman R., Gandevia S. C., Burke D. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol. 1991;440:497–512. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield V. G., Gandevia S. C., Bigland-Ritchie B., Gorman R. B., Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol. 1993 Nov;471:429–443. doi: 10.1113/jphysiol.1993.sp019908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey D. I., Gandevia S., Potter E. K., Colebatch J. G. Muscle sense and effort: motor commands and judgments about muscular contractions. Adv Neurol. 1983;39:151–167. [PubMed] [Google Scholar]
- McCloskey D. I., Torda T. A. Corollary motor discharges and kinaesthesia. Brain Res. 1975 Dec 19;100(2):467–470. doi: 10.1016/0006-8993(75)90503-x. [DOI] [PubMed] [Google Scholar]
- Melzack R., Bromage P. R. Experimental phantom limbs. Exp Neurol. 1973 Mar-Apr;39(2):261–269. doi: 10.1016/0014-4886(73)90228-8. [DOI] [PubMed] [Google Scholar]
- Meunier S., Pierrot-Deseilligny E. Gating of the afferent volley of the monosynaptic stretch reflex during movement in man. J Physiol. 1989 Dec;419:753–763. doi: 10.1113/jphysiol.1989.sp017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell J. C., Traub M. M., Day B. L., Obeso J. A., Thomas P. K., Marsden C. D. Manual motor performance in a deafferented man. Brain. 1982 Sep;105(Pt 3):515–542. doi: 10.1093/brain/105.3.515. [DOI] [PubMed] [Google Scholar]
- Sanes J. N., Mauritz K. H., Dalakas M. C., Evarts E. V. Motor control in humans with large-fiber sensory neuropathy. Hum Neurobiol. 1985;4(2):101–114. [PubMed] [Google Scholar]
- Tarkka I. M. Changes in the probability of firing of motor units following electrical stimulation in human limb muscles. Acta Physiol Scand. 1986 Jan;126(1):61–65. doi: 10.1111/j.1748-1716.1986.tb07789.x. [DOI] [PubMed] [Google Scholar]



