Abstract
1. The high-voltage-activated L-type calcium channel is a multi-protein complex of alpha 1, alpha 2/delta, beta and gamma subunits. The alpha 1 subunit contains the voltage-dependent calcium-conducting pore. Chinese hamster ovary (CHO) cells were stably transfected with the complementary DNA of the alpha 1, beta and alpha 2/delta subunits. These subunits were not detected in wild-type CHO cells. 2. The alpha 1 (CaCh2b) subunit itself directed the expression of functional calcium channels which bound calcium channel blockers and showed voltage-dependent activation and inactivation. 3. The co-expression of the alpha 1 subunit with the beta subunit (CaB1 gene) enhanced the density of the dihydropyridine binding sites 2- to 3-fold and increased dihydropyridine-sensitive barium inward currents (IBa) up to 3.5-fold from -13.3 microA/cm2 (alpha 1 subunit) to -46.7 microA/cm2 (alpha 1 and beta subunits). 4. Co-expression of the beta subunit did not change the sensitivity of IBa towards dihydropyridines, but accelerated current activation and inactivation and shifted the half-maximal steady-state activation and inactivation to slightly more hyperpolarizing potentials. 5. The co-expression of the alpha 2/delta subunit together with alpha 1 and beta subunits accelerated the inactivation kinetics of the channel without a major effect on the other parameters. 6. These results indicate that the beta and alpha 2/delta subunit interact with the alpha 1 subunit and modulate thereby the properties of the alpha 1 subunit-dependent inward current.
Full text
PDF



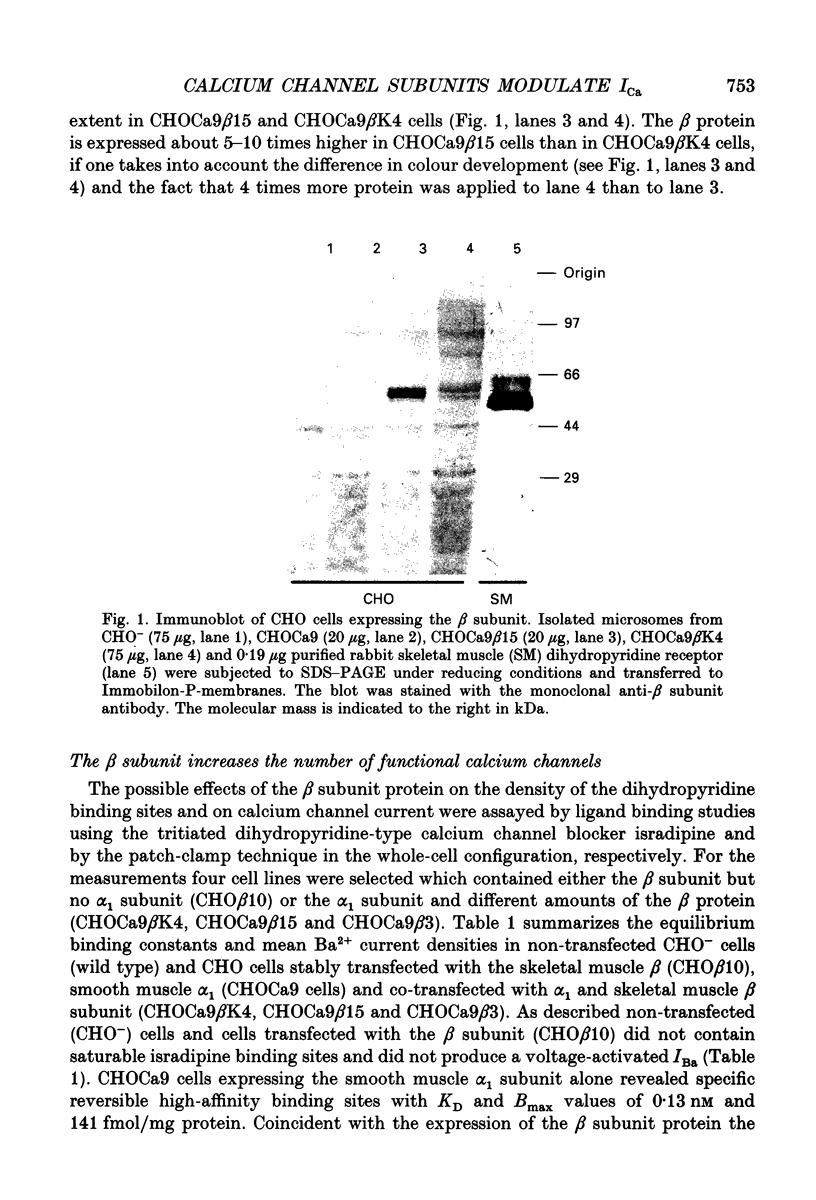
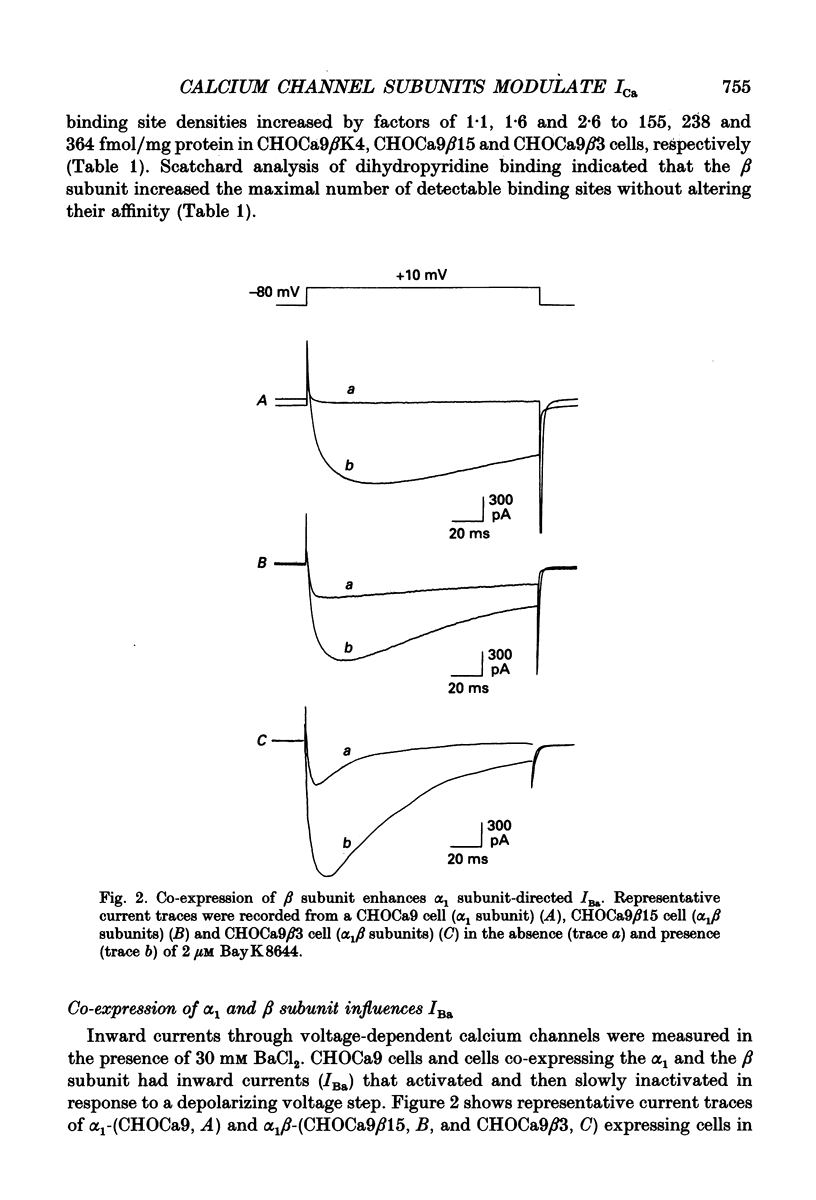
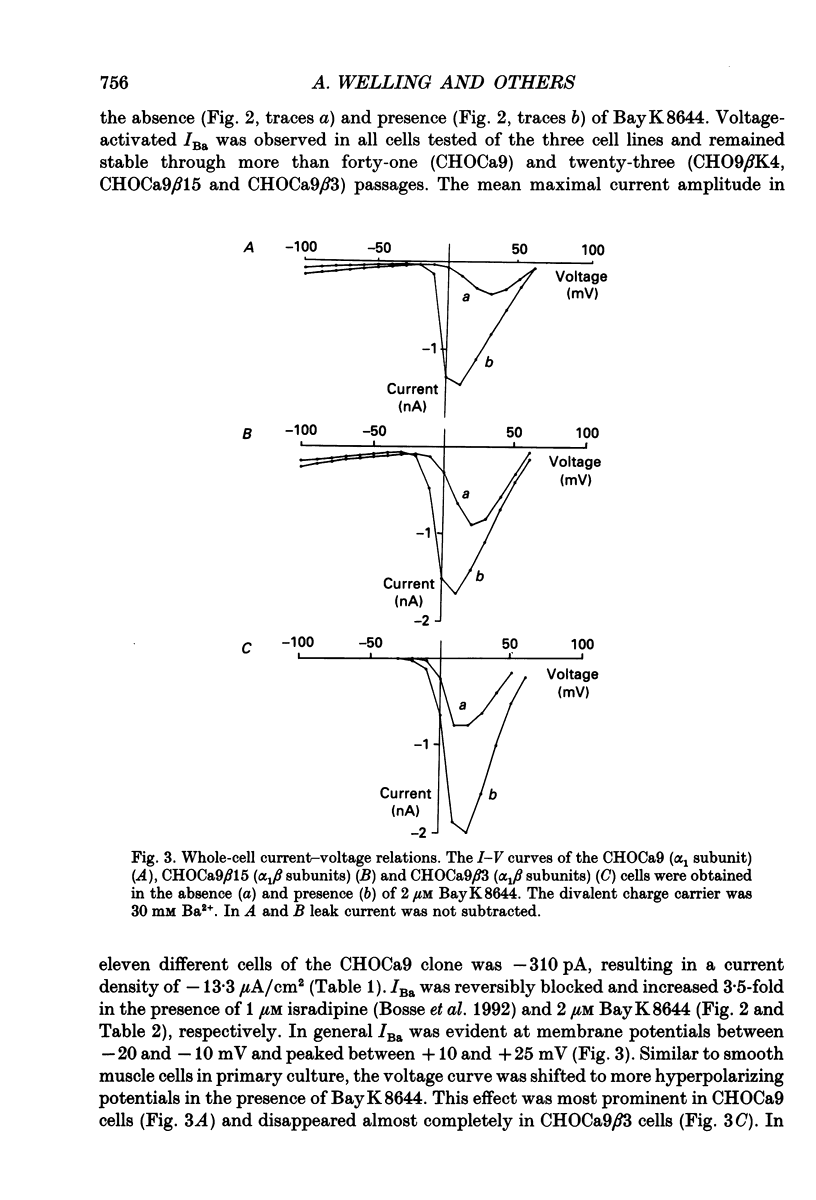
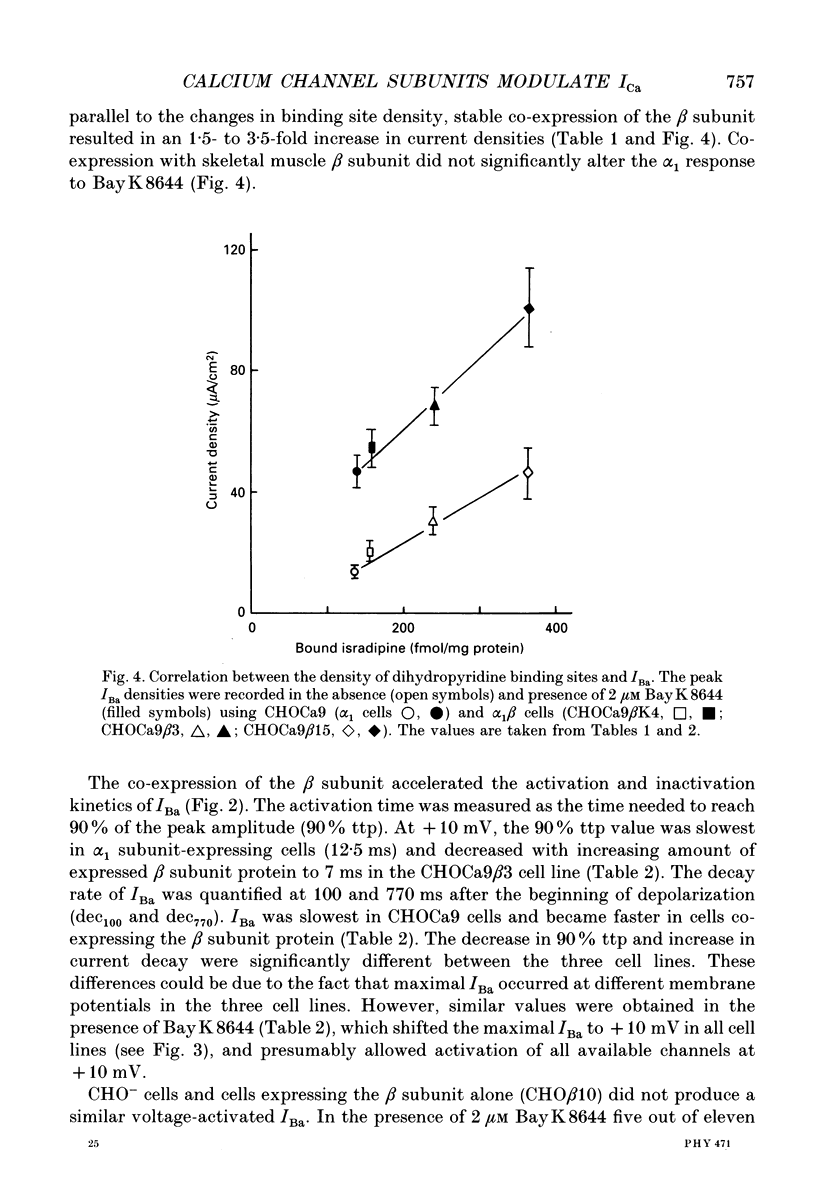
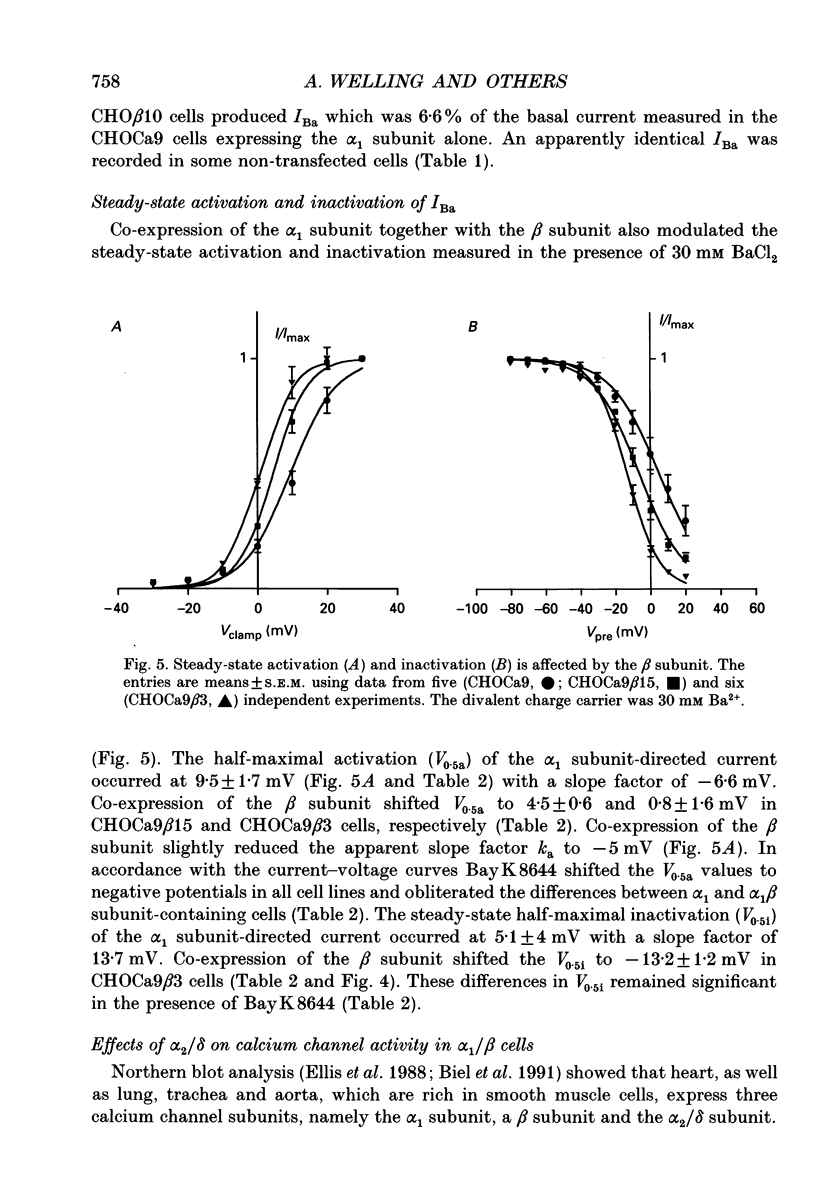
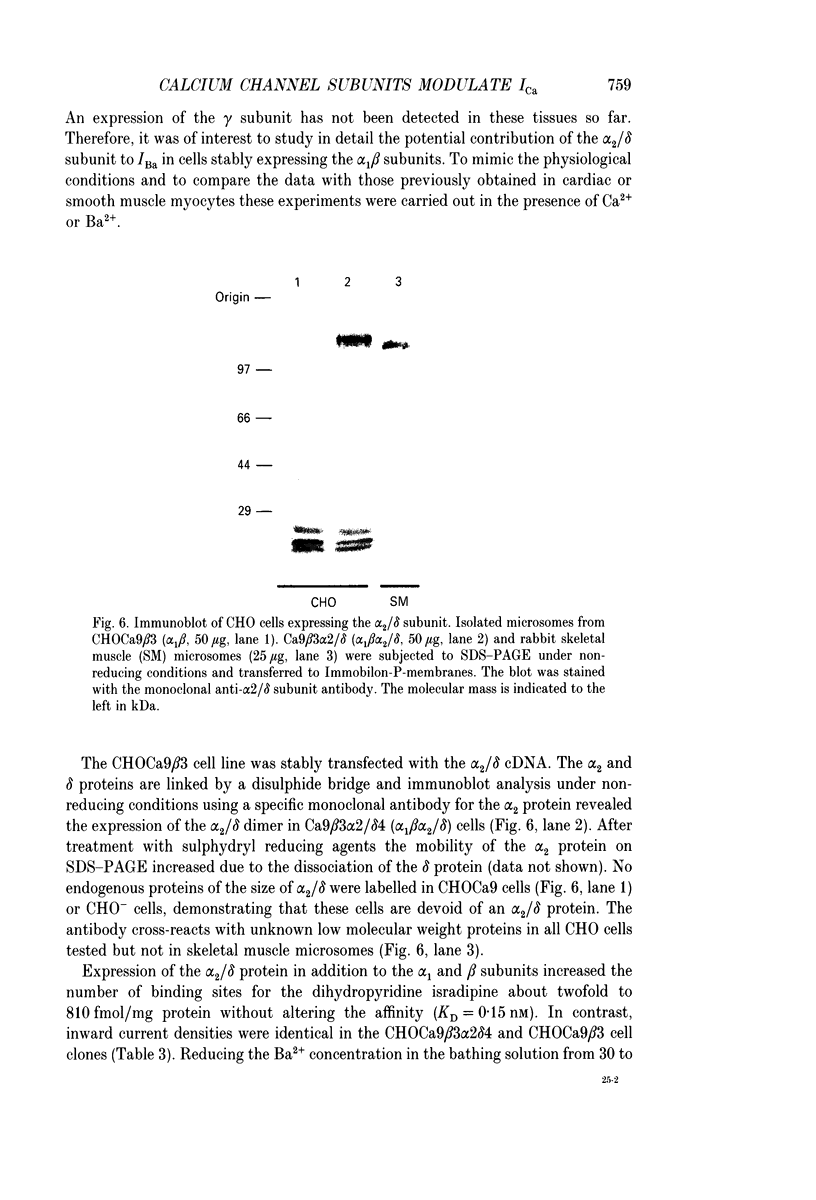
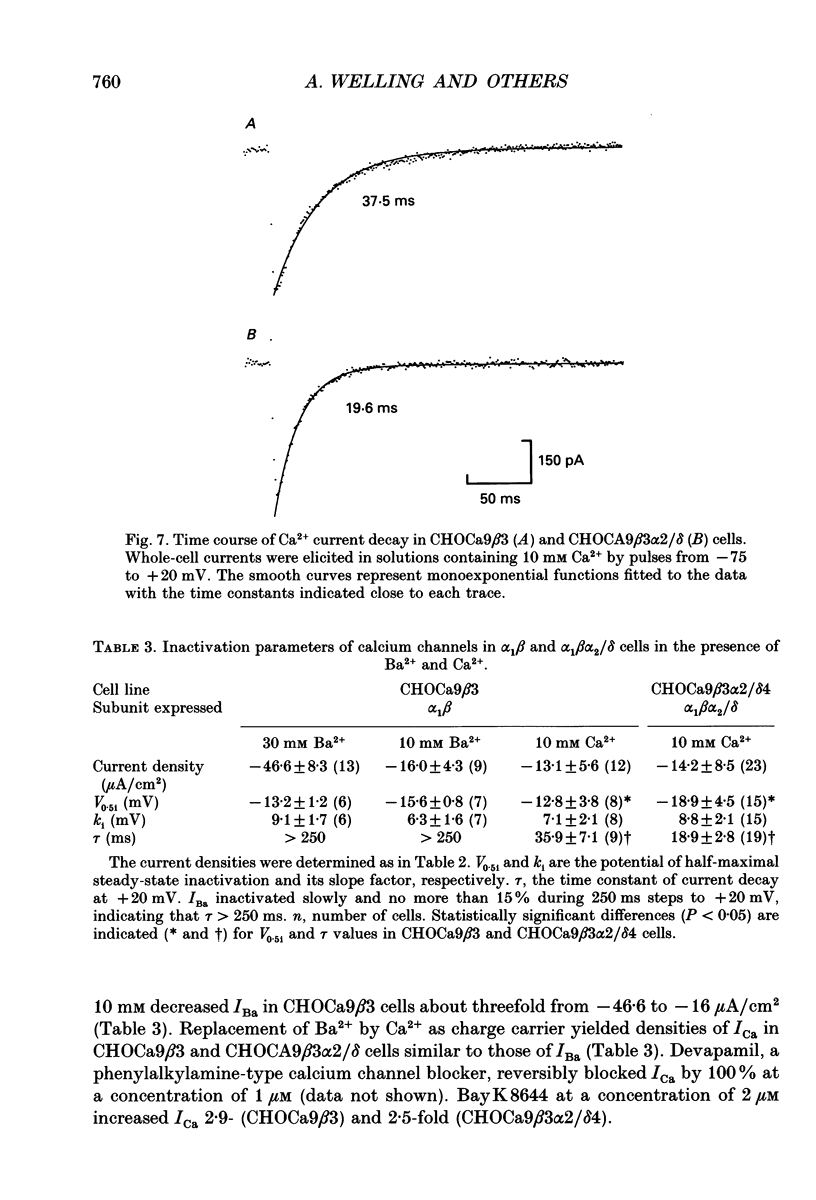
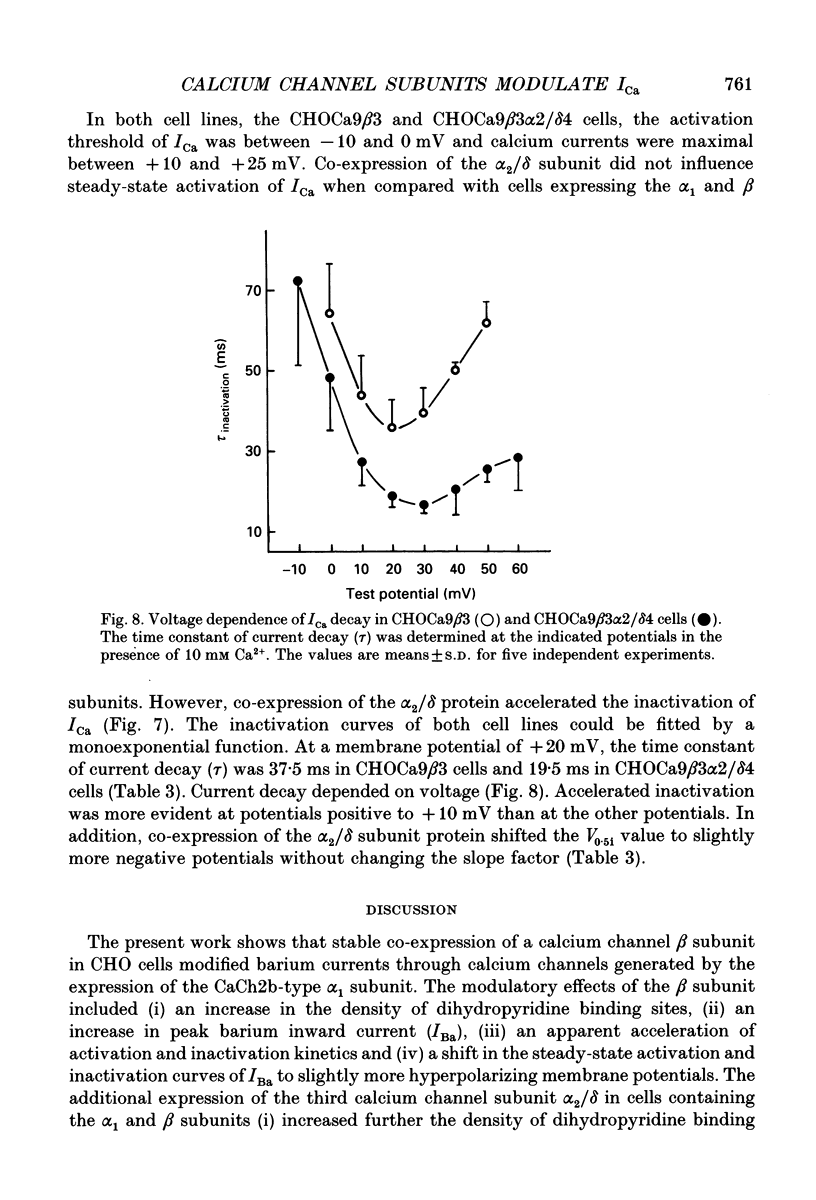




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beam K. G., Adams B. A., Niidome T., Numa S., Tanabe T. Function of a truncated dihydropyridine receptor as both voltage sensor and calcium channel. Nature. 1992 Nov 12;360(6400):169–171. doi: 10.1038/360169a0. [DOI] [PubMed] [Google Scholar]
- Biel M., Hullin R., Freundner S., Singer D., Dascal N., Flockerzi V., Hofmann F. Tissue-specific expression of high-voltage-activated dihydropyridine-sensitive L-type calcium channels. Eur J Biochem. 1991 Aug 15;200(1):81–88. doi: 10.1111/j.1432-1033.1991.tb21051.x. [DOI] [PubMed] [Google Scholar]
- Biel M., Ruth P., Bosse E., Hullin R., Stühmer W., Flockerzi V., Hofmann F. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS Lett. 1990 Sep 3;269(2):409–412. doi: 10.1016/0014-5793(90)81205-3. [DOI] [PubMed] [Google Scholar]
- Bosse E., Bottlender R., Kleppisch T., Hescheler J., Welling A., Hofmann F., Flockerzi V. Stable and functional expression of the calcium channel alpha 1 subunit from smooth muscle in somatic cell lines. EMBO J. 1992 Jun;11(6):2033–2038. doi: 10.1002/j.1460-2075.1992.tb05260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse E., Regulla S., Biel M., Ruth P., Meyer H. E., Flockerzi V., Hofmann F. The cDNA and deduced amino acid sequence of the gamma subunit of the L-type calcium channel from rabbit skeletal muscle. FEBS Lett. 1990 Jul 2;267(1):153–156. doi: 10.1016/0014-5793(90)80312-7. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Lotan I., Karni E., Gigi A. Calcium channel currents in Xenopus oocytes injected with rat skeletal muscle RNA. J Physiol. 1992 May;450:469–490. doi: 10.1113/jphysiol.1992.sp019137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh K. S., Warner C., Colvin A. A., Catterall W. A. Characterization of the two size forms of the alpha 1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S. B., Williams M. E., Ways N. R., Brenner R., Sharp A. H., Leung A. T., Campbell K. P., McKenna E., Koch W. J., Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988 Sep 23;241(4873):1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Hanukoglu I. Unraveling the structure of the intermediate filaments. Cell. 1983 Sep;34(2):332–334. doi: 10.1016/0092-8674(83)90367-7. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartman S. C., Mulligan R. C. Two dominant-acting selectable markers for gene transfer studies in mammalian cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullin R., Singer-Lahat D., Freichel M., Biel M., Dascal N., Hofmann F., Flockerzi V. Calcium channel beta subunit heterogeneity: functional expression of cloned cDNA from heart, aorta and brain. EMBO J. 1992 Mar;11(3):885–890. doi: 10.1002/j.1460-2075.1992.tb05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay S. D., Ellis S. B., McCue A. F., Williams M. E., Vedvick T. S., Harpold M. M., Campbell K. P. Primary structure of the gamma subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1990 Apr 27;248(4954):490–492. doi: 10.1126/science.2158672. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Itagaki K., Bodi I., Schwartz A. Beta-subunit expression is required for cAMP-dependent increase of cloned cardiac and vascular calcium channel currents. Pflugers Arch. 1992 Mar;420(3-4):413–415. doi: 10.1007/BF00374479. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Kim H. S., Ruth P., Perez-Reyes E., Flockerzi V., Hofmann F., Birnbaumer L., Brown A. M. Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature. 1991 Aug 8;352(6335):527–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory P., Varadi G., Schwartz A. The beta subunit controls the gating and dihydropyridine sensitivity of the skeletal muscle Ca2+ channel. Biophys J. 1992 Nov;63(5):1421–1424. doi: 10.1016/S0006-3495(92)81705-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Mori Y., Friedrich T., Kim M. S., Mikami A., Nakai J., Ruth P., Bosse E., Hofmann F., Flockerzi V., Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991 Apr 4;350(6317):398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Nastainczyk W., Ludwig A., Hofmann F. The dihydropyridine-sensitive calcium channel of the skeletal muscle: biochemistry and structure. Gen Physiol Biophys. 1990 Aug;9(4):321–329. [PubMed] [Google Scholar]
- Nukada T., Mishina M., Numa S. Functional expression of cloned cDNA encoding the alpha-subunit of adenylate cyclase-stimulating G-protein. FEBS Lett. 1987 Jan 19;211(1):5–9. doi: 10.1016/0014-5793(87)81263-2. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Kim H. S., Lacerda A. E., Horne W., Wei X. Y., Rampe D., Campbell K. P., Brown A. M., Birnbaumer L. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989 Jul 20;340(6230):233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Wei X. Y., Castellano A., Birnbaumer L. Molecular diversity of L-type calcium channels. Evidence for alternative splicing of the transcripts of three non-allelic genes. J Biol Chem. 1990 Nov 25;265(33):20430–20436. [PubMed] [Google Scholar]
- Powers P. A., Liu S., Hogan K., Gregg R. G. Skeletal muscle and brain isoforms of a beta-subunit of human voltage-dependent calcium channels are encoded by a single gene. J Biol Chem. 1992 Nov 15;267(32):22967–22972. [PubMed] [Google Scholar]
- Ruth P., Röhrkasten A., Biel M., Bosse E., Regulla S., Meyer H. E., Flockerzi V., Hofmann F. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989 Sep 8;245(4922):1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., McCleskey E. W., Almers W. Dihydropyridine receptors in muscle are voltage-dependent but most are not functional calcium channels. 1985 Apr 25-May 1Nature. 314(6013):747–751. doi: 10.1038/314747a0. [DOI] [PubMed] [Google Scholar]
- Singer D., Biel M., Lotan I., Flockerzi V., Hofmann F., Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991 Sep 27;253(5027):1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Varadi G., Lory P., Schultz D., Varadi M., Schwartz A. Acceleration of activation and inactivation by the beta subunit of the skeletal muscle calcium channel. Nature. 1991 Jul 11;352(6331):159–162. doi: 10.1038/352159a0. [DOI] [PubMed] [Google Scholar]
- Wei X. Y., Perez-Reyes E., Lacerda A. E., Schuster G., Brown A. M., Birnbaumer L. Heterologous regulation of the cardiac Ca2+ channel alpha 1 subunit by skeletal muscle beta and gamma subunits. Implications for the structure of cardiac L-type Ca2+ channels. J Biol Chem. 1991 Nov 15;266(32):21943–21947. [PubMed] [Google Scholar]
- Williams M. E., Feldman D. H., McCue A. F., Brenner R., Velicelebi G., Ellis S. B., Harpold M. M. Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron. 1992 Jan;8(1):71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]