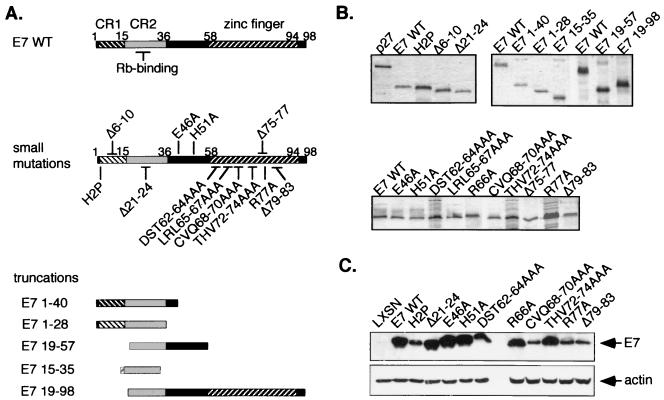
FIG. 1.
Expression of E7 proteins. (A) Locations of E7 mutations. CR1 and 2 and the C-terminal zinc finger are indicated on E7 WT. Substitution mutations are referred to by the residue changed, the position and the new residue (e.g., His 51 to Ala is H51A). Deletions are indicated by a “Δ” followed by the residues deleted. Truncated E7 proteins are named for the sequences remaining. Substitution mutations in the C terminus and truncations of N- and C-terminal sequences were generated as described in Materials and Methods. The C-terminal deletion mutations were provided by Massimi et al. (47). Representative CR1 and CR2 mutations were included in the study. (B) Recombinant E7 proteins for in vitro studies. Expression of bacterially produced His-tagged E7 proteins was confirmed by SDS-PAGE and Coomassie blue staining. (C) Expression of E7 proteins in keratinocytes. Expression of E7 proteins with small substitution or deletion mutations was confirmed in cells by immunoprecipitation and Western blotting with anti-E7 antibodies. Postimmunoprecipitation lysates were immunoblotted with an actin antibody as a control for lysate input.