Abstract
Background
Sentinel lymph node biopsy (SLNB) reduces the morbidity of axillary clearance and is the standard of care for patients with clinically node-negative breast cancer. The ability to analyse the sentinel node during surgery enables a decision to be made whether to proceed to full axillary clearance during primary surgery, thus avoiding a second procedure in node-positive patients.
Methods
Current evidence for intraoperative sentinel node analysis following SLNB in breast cancer was reviewed and evaluated, based on articles obtained from a MEDLINE search using the terms ‘sentinel node’, ‘intra-operative’ and ‘breast cancer’.
Results and conclusion
Current methods for evaluating the sentinel node during surgery include cytological and histological techniques. Newer quantitative molecular assays have been the subject of much recent clinical research. Pathological techniques of intraoperative SLNB analysis such as touch imprint cytology and frozen section have a high specificity, but a lower and more variably reported sensitivity. Molecular techniques are potentially able to sample a greater proportion of the sentinel node, and could have higher sensitivity.
An expanding technique
Introduction
Completion of the NEW START sentinel lymph node training programme in December 20081 has allowed sentinel lymph node biopsy (SLNB) to become standard practice in the UK for early node-negative breast cancer, as recommended by current National Institute for Health and Clinical Excellence guidelines2. This change in the staging and management of the axilla means that approximately 25 000 women each year are spared more extensive axillary surgery1.
The drive for less invasive management of the breast and axilla followed the success of national screening programmes in identifying breast cancer at an earlier stage. Less radical treatment of the breast in these patients was possible without detriment to long-term outcome. Similarly, less invasive management of the axilla was proposed in selected patients to avoid the morbidity of axillary clearance.
The histological status of the sentinel lymph node accurately reflects the overall status of the axilla in 97 per cent of cases3–7. Furthermore, avoidance of full axillary clearance on the basis of sentinel node staging does not increase the likelihood of axillary recurrence8–10. The Axillary Lymphatic Mapping Against Nodal Axillary Clearance (ALMANAC) trial compared 1031 patients with clinically node-negative breast cancer randomly assigned to one of two treatment pathways: 516 received primary axillary clearance or axillary sampling and 515 underwent SLNB with a delayed clearance or radiotherapy to the axilla when biopsy indicated nodal spread11. The trial demonstrated significantly reduced rates of lymphoedema and neuropathy, improved functional outcome and reduced hospital stay in the SLNB group, without a negative impact on patients' anxiety levels.
However, 25–30 per cent of patients undergoing SLNB will have a positive finding on biopsy5,11. Delayed axillary clearance as a second procedure following SLNB increases operating time and the duration of hospital stay12. This impact on bed occupancy and other health economic factors has driven research into intraoperative techniques for evaluating the status of sentinel lymph nodes. Accurate intraoperative detection of sentinel node metastasis would allow axillary clearance to be undertaken immediately during the primary procedure when the sentinel node is involved, thereby avoiding a second hospital admission and general anaesthetic.
In 1999 the College of American Pathologists recommended the use of cytological methods to evaluate the sentinel node during surgery13. Since then, a plethora of research has been published on the use of histological, cytological and molecular diagnostic assays in staging the sentinel node. Recent coverage in the popular press14 and a UK National Health Service initiative to facilitate national adoption of molecular techniques for intraoperative sentinel node analysis15 have raised the profile of this debate. The present paper reviews current evidence evaluating the efficacy of histological, cytological and molecular techniques.
Methods
The MEDLINE database was searched using the terms ‘sentinel node’, ‘intra-operative’ and ‘breast cancer’. All abstracts from English language articles and foreign language articles available in a translated form were examined by a single reviewer. Papers detailing relevant experimental data were assessed for quality independently by two separate reviewers. All review articles, systematic reviews and meta-analyses were assessed, and references of such articles were searched for additional relevant papers.
Papers that outlined their methodology sufficiently to allow comparison were included; articles that failed to detail the sectioning procedure of both the experimental technique and permanent histological control were excluded. Values for accuracy, sensitivity and specificity are given on a per-patient basis (unless stated otherwise) for reasons of clarity, because this was the most universally adopted format of data reporting. Where sufficient data were reported in the articles, values specific to macrometastasis and micrometastasis were derived, if not directly quoted by the original paper.
Current practice
Variation in local histological practice makes comparison of research data from different centres problematic. A pan-European survey of current practice within 240 units processing sentinel node biopsies demonstrated 123 different protocols in use16. Intraoperative assessment of SLNB was performed in 145 units (60·4 per cent). Of these, 101 (69·7 per cent) used frozen section in isolation, with a further 28 units employing a combination of imprint cytology and frozen section. Only 11·0 per cent of units used imprint cytology alone. Intraoperative immunohistochemistry (IHC) was performed in 9·7 per cent of laboratories. Further inconsistency was noted in the number of levels examined during surgery, with approximately 50 per cent of centres analysing a single level and 50 per cent examining multiple levels.
Variation in the reporting categories of lymph node metastasis size adds further complexity. For the purpose of this review, the following terms are used, as defined by current American Joint Committee on Cancer tumour node metastasis staging guidelines17: isolated tumour cells, single cells or small clusters of cells no greater than 0·2 mm in largest dimension; micrometastasis, tumour deposits larger than 0·2 mm but smaller than 2 mm in largest dimension; macrometastasis, tumour deposits greater than 2 mm in largest dimension.
Histological and cytological techniques
Current protocols employ frozen section, imprint and scrape cytology, rapid immunocytochemistry and combinations thereof in the intraoperative evaluation of sentinel nodes16.
Frozen section
Frozen section is the most commonly used technique. Its reported sensitivity in published literature ranges from 57 to 74 per cent18–24. Protocols for intraoperative frozen-section analysis and formalin-fixed paraffin-embedded ('permanent') sectioning vary widely, making comparison between studies difficult. Table 1 details the outcome of studies where comparison was made between frozen section and final histological staging of the sentinel node; studies where detail on protocol used was incomplete have been excluded.
Table 1.
Published studies on the use of intraoperative frozen-section analysis of sentinel lymph node biopsies where number of levels examined in intraoperative and permanent histology was specified in methodology
| Reference | No. of patients | SLNB-guided AXCL? | No. of SLNs examined | Frozen-section methods | Permanent staining methods | Accuracy (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|---|
| Veronesi et al.18 (1997) | 107 | All patients treated with AXCL | NS | SLN bisected if > 5 mm; 3 levels from one half; H&E stain | Paraffin; 3 levels from one half; H&E stain | Total 83 | Total 64 | Total 100 |
| Weiser et al.19 | 890 | Intraoperative, | NS | Single level; H&E | Paraffin; half node | Total 89 | Total 58 | Total 99 |
| (2000) | SLNB guided | stain | section at 50 µm; | Macro 92 | ||||
| 3 sections H&E | Micro 17 | |||||||
| stain and 2 | ||||||||
| sections IHC | ||||||||
| (CAM5·2 AE1/AE3) | ||||||||
| Rahusen et al.20 | 100 | Intraoperative, | 160 | SLN bisected if < 10 | Paraffin; initial single | Total 85 | Total 57 | Total 100 |
| (2000) | SLNB guided | mm; if > 10 mm, | level; if negative, | Macro 84 | ||||
| 5-mm sections; | additional 4 levels; | Micro 27 | ||||||
| single level from | H&E stain; IHC | |||||||
| each section | (CAM5·2) | |||||||
| Zurrida et al.21 (2000) | 192 | All patients treated with AXCL | NS | Bisected; 3 levels taken from one half | Paraffin; 3 levels from each half; H&E stain | Total 86 | Total 68 | Total 100 |
| Tanis et al.22 (2001) | 262 | Intraoperative, SLNB guided | 444 | Bisected; single level; H&E stain | Paraffin; H&E stain from 3 levels; IHC from 1 level (CAM5·2) | Total 90 | Total 74 | Total 99 |
| Van de Vrande | 615 | Intraoperative, | 994 | SLN bisected if > 5 | Paraffin serial | Total 90·7 | Total 71·6 | Total 100 |
| et al.23 (2009) | SLNB guided | mm; single level | section at 150 µm; | Macro 84·0 | ||||
| from one half; H&E | H&E stain; IHC | Micro 61·1 | ||||||
| stain | (CK-8) | |||||||
| Viale et al.24 (1999) | 155 | All patients treated with AXCL | 203 | Serial sections at 50-µm intervals; H&E stain and IHC* | None | NA | NA | NA |
SLN(B), sentinel lymph node (biopsy); AXCL, axillary clearance; NS, not specified; H&E, haematoxylin and eosin; IHC, immunohistochemistry; macro, macrometastases; micro, micrometastases; CK, cytokeratin; NA, not applicable.
IHC methodology: rapid staining (EPOS anti-cytokeratin/HRP; Dako, Copenhagen, Denmark) with MNF116 monoclonal antibody.
Predictably, when frozen section is compared with formal histology, greater concordance is reported by studies where the protocol for frozen section involves more extensive examination of the node. The specificity reported in all studies consistently approached 100 per cent, indicating that, despite variation in reported false-negative rates, the false-positive rate with frozen section is close to zero.
Frozen section is expensive, labour intensive and operator dependent, requiring a skilled biomedical scientist and dedicated histopathologist for each surgical session. Frozen sections are morphologically inferior to paraffin sections (Fig. 1) and may miss subtle lymph node metastases, particularly in lobular carcinoma, where the cells are usually cytologically bland and have an infiltrative growth pattern (Fig. 2). Furthermore, the process of cutting a frozen section results in irreversible tissue loss. Therefore, there is a theoretical potential for understaging of sentinel nodes when evidence of micrometastatic disease is corrupted by the frozen-section process. Unfortunately it is impossible to determine accurately the frequency of such an error through direct comparison. These problems with frozen section make an alternative desirable.
Fig. 1.
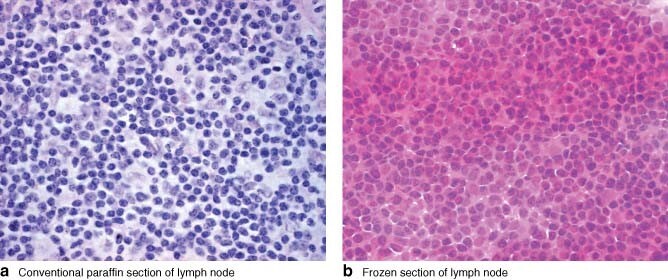
Comparison of the quality of paraffin and frozen sections. a Conventional paraffin section and b frozen section of the same lymph node (haematoxylin and eosin stain, original magnification × 600). The nuclear and cytoplasmic detail is seen more clearly on the paraffin section. The nuclear detail is obscured in the frozen section and the cytoplasm appears abnormally prominent
Fig. 2.
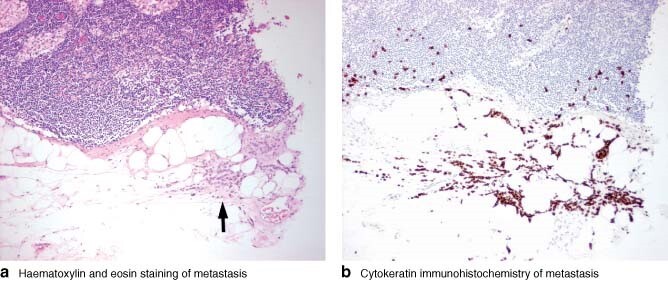
Lymph node containing a subtle metastasis from a lobular carcinoma. a On haematoxylin and eosin staining (original magnification × 100), an infiltrate of small, bland cells can be seen in the extranodal fat (arrow). b Cytokeratin immunohistochemistry (MNF116 × 100) highlights the more extensive nature of the metastasis, with single cells seen infiltrating into the node and more widely in the extranodal fat. It is highly likely that this subtle metastasis would have been missed on frozen section
Intraoperative cytology
Cytological techniques such as intraoperative imprint and scrape cytology have some technical advantages over frozen-section analysis. The cut surface of the sentinel node is pressed or scraped on to a glass slide, stained and examined. The preparation time and cost of cytological specimens is less than for frozen section, and there is no loss of tissue. Disadvantages include the small number of cells analysed, the significant expertise required to interpret cytological material and the potential for an inconclusive report that fails to guide intraoperative decisions.
In 2005, Tew and colleagues25 published a meta-analysis of 31 articles on the use of touch imprint cytology in sentinel node staging. Heterogeneity of methodology again makes these data difficult to interpret; within the 31 studies, there were differences in intraoperative assessment (6 different techniques), sectioning method (11 distinct protocols), imprint staining used (9 different stains used in various combinations), application of rapid IHC and immunofluorescence techniques (used in 7 of the 31 studies) and final staining method (3 distinct protocols). A random-effects model pooled estimate of the sensitivity of imprint cytology was 63 (95 per cent confidence interval (c.i.) 57 to 69) per cent and the specificity was 99 (98 to 99) per cent.
A significant variation continues to exist in the reported sensitivity of cytological techniques. Since the publication of Tew and co-workers25, further studies of imprint cytology have been published. Table 2 details their methods and results, with sensitivity ranging from 33 to 73 per cent and specificity of 98–100 per cent25–29.
Table 2.
Use of imprint cytology to stage sentinel nodes. Summary data from Tew et al.25 alongside papers not incorporated in the meta-analysis
| No. of patients | Touch imprint methods | Permanent section methods | Accuracy (%) | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|
| Pooled data from 31 | 4438 | Various | Various | Total 63 | Total 99 | |
| studies included in | Macro 81 | |||||
| Tew et al.25 | Micro 22 | |||||
| Barranger et al.26 | 180 | Bisected; Diff-Quick | 3-mm sections, each | Total 79 | Total 33 | Total 98 |
| stain | analysed 4 times; | Macro 75 | ||||
| 150-µm levels; | ||||||
| H&E + IHC (AE1–AE3) | ||||||
| Chicken et al.27 | 133 | Bisected; Giemsa stain | Sections at 3 levels; | Total 95 | Total 73 | Total 100 |
| H&E + IHC (AE1/AE3) | ||||||
| Cox et al.28 | 2137 | Bisected; Diff-Quick | Single section; further | Total 85 | Total 53 | Total 99 |
| stain | sections taken if initial | Macro 69·3 | ||||
| section negative; | Micro 6·4 | |||||
| H&E + IHC (CK) | ||||||
| Contractor et al.29 | 896 | Bisected; H&E stain | Single section; H&E stain | Total 92·5 | Total 73 | Total 100 |
Macro, macrometastasis; micro, micrometastasis; H&E, haematoxylin and eosin; IHC, immunohistochemistry; CK, cytokeratin.
False-negative results in imprint cytology are more common in the presence of micrometastatic disease25,30 and in invasive lobular carcinoma28. Tew et al.25 estimated that imprint cytology detected macrometastasis in SLNB with 81 per cent sensitivity and micrometastasis with 22 per cent sensitivity. The size of micrometastasis and the small amount of cellular tissue examined combine to make their detection difficult by imprint cytology. Increasing the number of cut nodal surfaces sampled would increase the pick-up rate simply by increasing the total volume of the node examined. However, studies in which each node is sectioned extensively once formalin fixed and paraffin embedded are likely to find a lower sensitivity for imprint cytology as they identify micrometastatic disease with greater frequency during final histological examination.
Invasive lobular carcinoma presents an additional problem because its cells are usually of low histological grade, are poorly cohesive and may resemble lymphoid cells morphologically (Fig. 3). This makes their detection on cytological specimens difficult. Cox and colleagues28 reported a sensitivity of 38·7 per cent in identification of lobular carcinoma metastasis using imprint cytology, compared with 55·5 per cent in invasive ductal carcinoma.
Fig. 3.
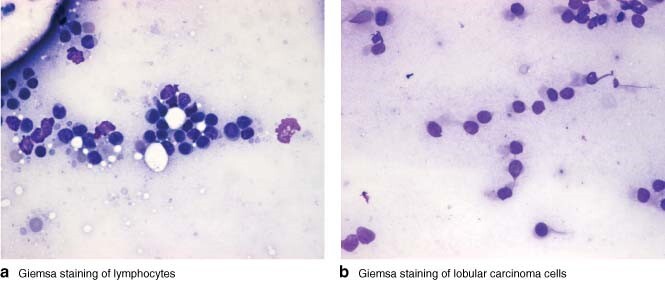
Comparison of a lymphocytes and b lobular carcinoma cells (both Giemsa stain, original magnification × 400). Both cell types have small, round, bland nuclei. The lobular carcinoma cells are subtly different, possessing more cytoplasm and having eccentrically located nuclei
Some authors have advocated the routine use of immunocytochemical techniques in intraoperative imprint cytology of specimens from patients with known invasive lobular carcinoma after demonstrating that this technique improves diagnosis markedly31. Similarly, the use of immunocytochemical techniques has also been demonstrated by some authors to improve the detection of micrometastasis on imprint slides32. In the meta-analysis by Tew et al.25, the pooled estimate of sensitivity in the seven studies that employed immunocytochemistry was 66 per cent, compared with a pooled sensitivity of 60 per cent in studies where immunocytochemistry was not used25. However, immunostaining has an uncertain role in intraoperative staging of SLNB; it is time consuming and expensive, making it less practical for intraoperative use.
Frozen section versus intraoperative cytology
Three of four studies33–36 comparing frozen section and imprint cytology found frozen section to have a greater sensitivity than imprint cytology33–35 (Fig. 4). The fourth study36 employed immunostaining and showed an advantage in the use of imprint cytology. Tew and co-workers25 estimated pooled sensitivity and specificity for frozen section to be 76 and 99 per cent respectively, compared with 63 and 99 per cent for imprint cytology. The small advantage reported in sensitivity for frozen section might well be overcome by increasing the number of slides taken during imprint cytology. Such an increase would improve sensitivity37 without the deleterious effect of losing tissue for formal histological examination, which remains the key advantage of imprint cytology over frozen section.
Fig. 4.
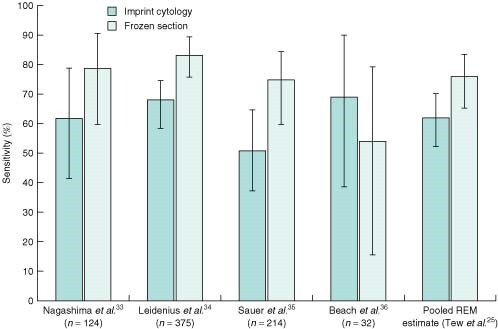
Results of four studies comparing the sensitivity of frozen section with that of imprint cytology. Error bars denote 95 per cent confidence intervals. REM, random effects model
Molecular techniques: quantitative reverse transcriptase–polymerase chain reaction and one-step nucleic acid amplification
Standard histological sampling protocols examine only a small proportion of the total volume of the sentinel node. This introduces the probability of significant sampling error in these techniques: a negative result might occur simply through failure to examine the part of the node that contains metastasis.
Molecular techniques have the potential to eliminate sampling error. The sample tissue is homogenized and scrutinized for the presence of marker genetic material. This potentially enables analysis of the entire node. As these techniques require the presence of only a single trained technician at the point of analysis, this increase in volume examined is attainable without greatly increasing the burden on the histopathology department.
Both quantitative reverse transcriptase–polymerase chain reaction (qRT–PCR) and one-step nucleic acid amplification (OSNA) have been proposed as viable techniques for intraoperative node analysis. They rely on detection of the mRNA for marker genes that are overexpressed in tumour cells but not in normal tissue.
Quantitative reverse transcriptase–polymerase chain reaction
Molecular diagnostics was proposed initially as a method for detecting tumour-specific antigens in peripheral blood, lymphatic tissue and bone marrow38–42. The presence of such antigens remains of uncertain prognostic significance but is the subject of ongoing research43,44. The value of molecular assays in the detection of lymph node metastasis was limited by the high sensitivity of the techniques; tumour mRNA markers, although expressed in neoplastic cells, are also present in normal tissues, albeit to a lesser extent45. Specificity was therefore too low to be of use in detecting metastases with qualitative techniques46–48.
Newer quantitative techniques, such as qRT–PCR, allow differentiation between the high levels of marker mRNA expressed by tumour cells and the low, legitimate expression by non-neoplastic tissues49,50. These techniques use fluorescence to calculate the quantity of target genetic material produced real-time during PCR. This is compared to a threshold level—the level that would be the upper limit of normal expression within non-neoplastic tissues. An expression above the threshold indicates the presence of metastasis.
The ideal marker for detection of sentinel node metastases would be expressed by 100 per cent of metastatic breast cancer cells but not by any non-neoplastic tissues, and be suitable for DNA probe design. Although some genes, such as those for cytokeratin (CK) 19 and mammaglobin (MGB) 1, are expressed by the vast majority of breast cancers, no one gene is expressed universally. This therefore limits the sensitivity of single-marker assays51. It is agreed that multigene assays increase sensitivity52,53; however, the use of too many markers might have a deleterious effect on the specificity of the assay.
The optimal number of markers is probably two or three, although which genes should be used remains controversial. Backus and colleagues52 compared molecular techniques with extensive histological sectioning under laboratory conditions. They achieved 91 per cent sensitivity and 97 per cent specificity using a combination of MGB1 and CK-19 markers. Hughes and co-workers53 estimated sensitivity in excess of 97 per cent when using either MGB1 and CK-19, or prolactin-inducible protein (PIP) and tumour-associated calcium signal transducer 1 markers53. However, three pseudogenes for CK-19 exist within the human genome, causing concern that, if RNA isolation is not complete before PCR, false positives with CK-19-based assays are a possibility53.
The first commercially available qRT–PCR assay for intraoperative assessment of sentinel node material was the GeneSearch™ Breast Lymph Node (BLN) Assay (Veridex, Warren, New Jersey, USA). The assay kit provides standard reagents, controls and detailed protocols, which allow maximum reproducibility within and between laboratories. It relies on the use of MGB1 and CK-19 in a dual-marker assay. A positive assay is one where the expression of either marker exceeds a threshold level, calibrated to correlate with the presence of metastases greater than 0·2 mm in diameter. The implementation of such technology has, however, been hindered by the imminent withdrawal of the commercial GeneSearch™ BLN assay. Reasons suggested include poorer than expected uptake in the USA, particularly in centres already running intraoperative pathological analysis such as frozen section, high start-up costs and continued uncertainty regarding the significance or otherwise of the low experimental specificity when compared to histological sectioning54. Despite this, the principle of molecular analysis through qRT–PCR techniques has been established, and work is under way on developing non-commercial open-access alternatives.
Eight papers have been published evaluating the application of qRT–PCR, with promising results (Table 3)55–62. The overall sensitivity of qRT–PCR was 78–96 per cent, exceeding that of imprint cytology and frozen section. Julian et al.55 directly compared qRT–PCR with frozen section in 319 patients and found a sensitivity (95 per cent c.i.) of 95·6 (89·0 to 98·8) and 85·6 (76·6 to 92·1) per cent respectively when using permanent histological sectioning as standard. qRT–PCR also appears to detect metastatic lobular carcinoma more effectively than histological techniques56.
Table 3.
Performance of quantitative reverse transcriptase–polymerase chain reaction in published literature
| No. of patients | Subgroup analysis | Sensitivity (%)* | Specificity (%)* | Agreement (%) | |
|---|---|---|---|---|---|
| Julian et al.55 and | 416 | Total 416 | 87·6 (80·4, 92·9) | 94·2 (90·9, 96·6) | 92·3 |
| Blumencranz et al.56† | Macro 94 | 97·9 (92·5, 99·7) | |||
| Micro 23 | 56·5 (34·5, 76·8) | ||||
| Lobular 57 | 80·0 | 91·9 | 87·7 | ||
| Viale et al.57 | 293 | Total 293 | 77·8 | 95·0 | 90·8 |
| Macro 52 | 98·1 | ||||
| Micro 20 | 25 | ||||
| Martinez et al.58 | 82 | Total 124 | 88·9 (56·5, 98·0) | 95·7 (90·2, 98·1) | 95·2 |
| Macro 6 | 100 | ||||
| Micro 3 | 66·7 | ||||
| Mansel et al.59 | 78 | Total 78 | 92 | 97 | 96 |
| Veys et al.60 | 367 | Total 367 | 89 | 94·5 | 93·5 |
| Tafe et al.61 | 59 | Total 59 | 88·9 (51·8, 99·7) | 93·5 (82·1, 98·6) | 86·4 |
| Cutress et al.62‡ | 254 | Total 256 | 96 | 95 | 95 |
| Macro | 100 |
Values in parentheses are 95 per cent confidence intervals.
References 55 and 56 grouped together because they involved the same patient group and only data from the validation cohort in each study were described;
data compared reverse transcriptase–polymerase chain reaction (RT–PCR) result with final axillary node status, which took into account non-sentinel nodes in cases where intraoperative RT–PCR was positive.
For all other references, data represent comparison of sentinel node status in RT–PCR versus histopathology. Macro, macrometastases; micro; micrometastases.
All studies compared molecular analysis with extensive sectioning. Unfortunately, because qRT–PCR requires homogenization of sample tissue, histological examination of the same tissue, and therefore direct comparison, was not possible. This leaves the potential for discrepancies due to sampling error. Such error may account for the lower specificity seen in qRT–PCR. False-positive results in qRT–PCR may occur when the metastatic deposit is entirely within that part of the node undergoing qRT–PCR analysis, so that it remains undetected by histological techniques. The converse may also, of course, be true, with sampling error erroneously reducing the apparent sensitivity of qRT–PCR.
Despite the undoubted significance of this sampling effect, the apparent lower specificity of molecular techniques does raise the question whether such assays are prone to true false-positive results. Further analysis of lysate from BLN-positive, histology-negative tumours with additional molecular markers B305D, B726, PIP and prostate-derived Ets transcription factor supported the presence of metastatic material in 73–76 per cent of these samples55,56. This suggests that the reported specificity was an underestimate of true specificity owing to histology sampling a smaller proportion of the node than the molecular assay.
In addition, each assay is run with a series of internal and external controls to protect from operator error or kit dysfunction producing false-positive or false-negative results. There does, however, remain a potential for contamination of node samples with breast tissue, which would result in false-positive assays. Rigorous surgical technique and minimizing the amount of extranodal tissue homogenized during sample preparation are necessary to reduce the risk of such contamination.
One-step nucleic acid amplification
OSNA, like qRT–PCR, is a molecular diagnostic technique used to detect target gene mRNA. It also uses reverse transcriptase to convert mRNA to cDNA; however, gene replication is by loop-mediated isothermal amplification (LAMP). This variation on PCR uses six primers specific to the same cDNA target. These primers are designed so that looping of the DNA occurs during the amplification phase. This releases pyrophosphate as a byproduct, which binds with magnesium and precipitates. The rate of precipitation, or turbidity, of the solution is used to quantify the amount of target gene present63.
The difference between RT–LAMP (OSNA) and qRT–PCR is that OSNA does not use the denaturation steps required in qRT–PCR (Fig. 5). In addition, because of the pH and temperature at which OSNA is run, there is no need for meticulous extraction of RNA from genomic DNA. The lysate is buffered at pH 3·5, which precipitates the vast majority of genomic DNA, and isothermal cycling at 65 °C is too cool for genomic DNA to denature. Therefore, only cDNA is available for the primers to bind to. The fact that six primers are required to bind the same gene also increases the assay's specificity. This means that OSNA is relatively immune from genomic pseudogene interference which is, as discussed above, a possible source of false-positive results in qRT–PCR, particularly for CK-1953.
Fig. 5.
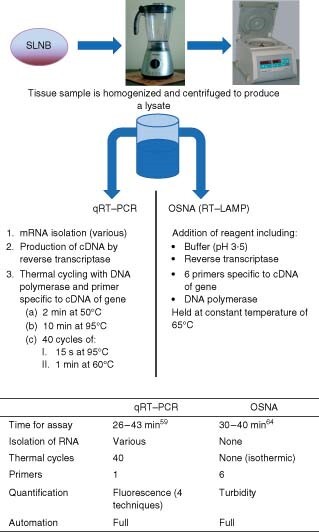
Methodology of quantitative reverse transcriptase–polymerase chain reaction (qRT–PCR) and one-step nucleic acid amplification (OSNA). SLNB, sentinel lymph node biopsy; RT–LAMP, reverse transcriptase–loop-mediated isothermal amplification
Tsujimoto and colleagues65 recently described a protocol for the use of OSNA in the detection of CK-19 mRNA within sentinel nodes. Concordance of extensive three-level histology with 2-mm sectioning, using IHC stains for CK-19, was 98·2 per cent. The assay also showed some ability to differentiate macrometastasis from micrometastasis. The same protocol was ratified by an independent group of researchers who examined 346 stored axillary nodes64. These authors demonstrated a sensitivity of 95·3 per cent and a specificity of 94·7 per cent for OSNA CK-19 using histology as a comparison (serial sectioning at 250-µm intervals; haematoxylin and eosin with CAM5·2 IHC staining)64. Both authors described the effect of sample error when comparing OSNA with histological sectioning.
Schem et al.66 examined 343 non-sentinel lymph nodes from patients undergoing completion axillary clearance who were identified before surgery as being node positive clinically or following a positive SLNB. The nodes were split into four sections and comparison was made between OSNA CK-19 and five-level histological sectioning with CK-19 and Lu5 IHC staining. Reported sensitivity on a per-node basis was 98·1 per cent; however, per-node specificity within the entire cohort was 89·0 per cent. When qRT–PCR and western blot techniques were carried out on the remaining lysate from OSNA-positive, histology-negative tumours, 11 of 26 samples were positive for markers of metastasis, suggesting that 89 per cent was an underestimate of the true specificity.
The results from a further Japanese multicentre study support this, demonstrating a specificity of 97·1 per cent (95 per cent c.i. 91·8 to 99·4) from 124 axillary nodes where OSNA was compared with extensive sectioning at 0·2 mm intervals with HE staining and IHC for CK-1979. However, when performance of OSNA was evaluated with a further 450 axillary nodes, using a “routine” histological sectioning protocol as comparison (three sections taken from the cut surface of the quartered node) the sensitivity was 87·7 per cent (95 per cent c.i. 78·5 to 93·9); overall agreement was 92·9 per cent (95 per cent c.i. 90·1 to 95·1).
Additional clinical studies are required to evaluate this emerging technique further.
Future methods
Elastic scattering spectroscopy (ESS) detects the abnormal cellular architecture present in metastatic disease through changes in light absorption and scattering properties. A probe interrogates tissues by emitting pulses of white light and collecting the backscattered signal. A computer then analyses the return signal for changes characteristic of tightly packed cellular constituents (nucleus, mitochondria) or abnormal relative size of these components.
Such probes are able to interrogate a volume of tissue 0·5 mm in diameter and 1 mm deep with each flash of light. This technique has been used in Barrett's oesophagus to differentiate between normal tissue, high-grade dysplasia and carcinoma67, and in SLNB to detect breast cancer metastasis68. This technology remains experimental and the first clinical trial results are just starting to be reported78.
ESS offers the possibility of intraoperative analysis of the sentinel node without the need for a specialist pathologist. Other potential advantages include minimal tissue preparation and destruction, instant results and low running costs. However, because the device can only analyse tissue of a maximal thickness of 1 mm, the same confounding sampling errors implicit in sectioning will apply as for histological analysis.
Discussion
A problem common to all histological methods of intraoperative staging is that any protocol used is a compromise between sensitivity and practicality. Comprehensive evaluation of a 2-cm node aimed at finding all metastatic disease more than 0·2 mm in size would require 100 sections. Viale et al.24 have described a protocol whereby the entire node is subjected to frozen section, with over 60 sections taken from each node. However, application of this technique to intraoperative analysis required a team of histopathologists in theatre to analyse material69, which is clearly beyond the means of most centres.
As qRT–PCR has the potential to reduce or eliminate sampling error, depending on the amount of tissue reserved for histological examination, it may provide a more sensitive assessment of the sentinel node than histology alone. A study by Weigelt and colleagues70 analysed 70 sentinel nodes staged as negative for metastasis by conventional histology. The qRT–PCR assay identified seven nodes as positive, four of which were found to contain micrometastases on further histological examination.
There is, however, an inherent error in attempting to validate molecular assays through comparison with histopathology; the tissue for qRT–PCR is homogenized and is therefore not available for histological examination. The two techniques never examine the same tissue and discrepancies due to sampling will occur. Similar discrepancies have been shown to occur in histological examination: 6 per cent of histological slides will be negative despite sections from adjacent tissue being positive55. Investigators have therefore argued that a 94 per cent concordance between molecular assays and histology is the maximum expected, the 6 per cent discrepancy in results being accounted for by sampling error.
Existing data suggest that molecular assays are more sensitive than frozen section and imprint cytology for the intraoperative analysis of sentinel lymph nodes. By identifying a higher proportion of sentinel node metastases, molecular assays would prevent a greater number of secondary axillary clearances. Cost analysis performed at a large UK district general hospital found that savings implicit in reducing numbers of secondary procedures, such as reduced bed and theatre occupancy, comfortably offset the expense of intraoperative RT–PCR for the health economy, although current tariff structures reduce the attractiveness to individual hospitals62.
Although molecular assays may potentially mitigate the strain on pathology services implicit in the introduction of intraoperative sentinel node assessment techniques, increased intraoperative sentinel node analysis may lead to difficulties in theatre scheduling. The impact would be minimized by increased preoperative axillary screening with ultrasonography and fine-needle aspiration, allowing node-positive patients to proceed directly to immediate axillary clearance. Stratification of clinically and radiologically node-negative axillae within theatre lists by criteria such as tumour size and grade, which are known to be predictive of the probability of node positivity71, might minimize the likelihood of the majority of patients on a single operating list requiring conversion to axillary clearance.
Clear explanation and adequate preoperative counselling undoubtedly play a vital role in the implementation of intraoperative SLNB analysis. The psychological effect on patients who undergo SLNB with preoperative uncertainty as to whether they will proceed to an axillary clearance merits further study, as does the impact of the small proportion of false-positive or false-negative intraoperative results.
It has been suggested that there is potential for molecular techniques to supplant formal histology as the standard method for detection of metastasis. Advantages include greater automation, analysis of a greater volume of the lymph node, the rapidity of such tests, financial savings and the objective nature of molecular diagnostics. The importance of objectivity should not be underestimated. Discordance between pathologists in the interpretation of slides can be considerable; one study showed that when ten independent pathologists looked at slides taken from sentinel node biopsies, 100 per cent agreement in interpretation occurred in just 12 per cent of cases72.
When using molecular techniques exclusively, histopathological markers of prognosis such as size of metastatic deposits and presence of extranodal or extracapsular spread would remain unrecognized. Loss of such important indicators, which are widely used to guide contemporary oncological practice, is a significant disadvantage. Furthermore, storage of histological samples allows cases to be reviewed years after the index presentation. Often only the histological features of the index primary metastasis can be used to differentiate between recurrence and a new focus of primary disease.
Introduction of molecular diagnostic techniques into clinical practice would increase the number of positive lymph node biopsies. Weigelt and co-workers70 suggested that RT–PCR might upstage at least 10 per cent of sentinel nodes, subsequently increasing the number of axillary clearances performed, yet the benefit of axillary clearance in patients with low-volume metastatic disease is unclear. The incidence of non-sentinel node disease is far greater in the presence of macrometastasis (63 per cent) compared with that present with micrometastases73. Meta-analysis of the reported incidence of non-sentinel node involvement in the presence of isolated micrometastasis within the sentinel node is 10–15 per cent, and falls to 9 per cent when sentinel node disease is only identifiable when IHC is used74. The presence of micrometastatic disease is generally considered a negative prognostic indicator75,76, although this remains controversial77 and not all studies have shown prognostic significance.
Intraoperative analysis of SLNB continues to evolve, while its application becomes more widespread. The question remains which technique will dominate future practice? Molecular-based techniques offer the greatest propensity for intraoperative diagnosis of low-volume metastatic disease, appearing to outperform histological techniques. They also provide an objective result quickly, are cost effective and do not invoke the expense of a dedicated pathologist. qRT–PCR techniques are also becoming increasingly prevalent in other areas of medicine, with the result that investment in equipment could be spread over several departments. This confers an advantage of qRT–PCR over OSNA which, at present, has far fewer additional clinical applications.
Although questions remain over the appropriate management of low-volume metastases within sentinel nodes, both qRT–PCR60 and OSNA65 are able to differentiate between micrometastatic and macrometastatic disease. Their use might therefore still be practical in centres where axillary dissection is reserved for patients with macrometastatic disease59.
Whether quantification of molecular markers of tumour cell metastases, such as CK-19 and MGB1, within sentinel node and other tissues provides an independent prognostic indicator in patients with breast cancer remains unclear. The establishment of such a link between levels of molecular markers and disease progression might conceivably allow molecular diagnostics to supersede formal histopathology. In the immediate future, however, it is likely that the two techniques will continue to be applied simultaneously.
Acknowledgements
R.I.C. is supported by Cancer Research UK. The authors declare no conflict of interest.
Contributor Information
D M Layfield, Southampton Breast Surgical Unit, Southampton University Hospitals Trust, Southampton, UK.
A Agrawal, Portsmouth Breast Surgical Unit, Portsmouth Hospitals NHS Trust, Portsmouth, UK.
H Roche, Department of Cellular Pathology, Southampton General Hospital, Southampton, UK.
R I Cutress, Southampton Breast Surgical Unit, Southampton University Hospitals Trust, Southampton, UK.
References
- 1. Mansel RE, MacNeill F. NEW START—Closing Remarks. The Association of Breast Surgery at BASO—Yearbook 2009; http://www.baso.org/Downloads/YearBook2009.pdf [accessed 12 November 2009].
- 2. National Institute for Health and Clinical Excellence (NICE) . Early and Locally Advanced Breast Cancer: Diagnosis and Treatment. NICE Clinical Guideline 80. NICE: London, 2009; http://www.nice.org.uk/nicemedia/pdf/CG80NICEGuideline.pdf [accessed 12 November 2009]. [Google Scholar]
- 3. Albertini JJ, Lyman GH, Cox C, Yeatman T, Balducci L, Ku N et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA 1996; 276: 1818–1822. [PubMed] [Google Scholar]
- 4. Veronesi U, Paganelli G, Galimberti V, Vialle G, Zurrida S, Bedoni M et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph nodes. Lancet 1997; 349: 1864–1867. [DOI] [PubMed] [Google Scholar]
- 5. Turner RR, Ollila DW, Krasne DL, Giuliano AE. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg 1997; 226: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnwell JM, Arredondo MA, Kollmorgen D, Gibbs JF, Lamonica D, Carson W et al. Sentinel node biopsy in breast cancer. Ann Surg Oncol 1998; 5: 126–130. [DOI] [PubMed] [Google Scholar]
- 7. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 2007; 8: 881–888. [DOI] [PubMed] [Google Scholar]
- 8. Naik AM, Fey J, Gemignani M, Heerdt A, Montgomery L, Petrek J et al. The risk of axillary relapse after sentinel node biopsy for breast cancer is comparable with that of axillary lymph node dissection: a follow-up study of 4008 procedures. Ann Surg 2004; 240: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Veronesi U, Galimberti V, Mariani L, Gatti G, Paganelli G, Viale G et al. Sentinel node biopsy in breast cancer: early results in 953 patients with negative sentinel node biopsy and no axillary dissection. Eur J Cancer 2005; 41: 231–237. [DOI] [PubMed] [Google Scholar]
- 10. Van der Ploeg IMC, Nieweg OE, van Rijk MC, Valdés Olmos RA, Kroon BB. Axillary recurrence after a tumour-negative sentinel node biopsy in breast cancer patients: a systematic review and meta-analysis of the literature. Eur J Surg Oncol 2008; 34: 1277–1284. [DOI] [PubMed] [Google Scholar]
- 11. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006; 98: 599–609. [DOI] [PubMed] [Google Scholar]
- 12. Goyal A, Newcombe RG, Chhabra A, Mansel RE. Morbidity in breast cancer patients with sentinel node metastasis undergoing delayed axillary lymph node dissection (ALND) compared with immediate ALND. Ann Surg Oncol 2008; 15: 262–267. [DOI] [PubMed] [Google Scholar]
- 13. Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM et al. Prognostic factors in breast cancer. College of American Pathologists consensus statement 1999. Arch Path Lab Med 2000; 124: 966–978. [DOI] [PubMed] [Google Scholar]
- 14. Rose D. ‘One-stop’ test for breast cancer to end agonising wait for result. The Times 31 October 2009; http://www.timesonline.co.uk/tol/life_and_style/health/article6897567.ece [accessed 5 November 2009].
- 15. NHS Technology Adoption Centre . Intra-operative Breast Lymph Node Assay. http://www.technologyadoptionhub.nhs.uk/?page_id=775 [accessed 12 February 2010].
- 16. Cserni G, Amendoeira I, Apostolikas N, Bellocq JP, Bianchi S, Boecker W et al. Discrepancies in current practice of pathological evaluation of sentinel lymph nodes in breast cancer. Results of a questionnaire based survey by the European Working Group for Breast Screening Pathology. J Clin Pathol 2004; 57: 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG et al. AJCC Cancer Staging Manual (6th edn). Springer: New York, 2002. [Google Scholar]
- 18. Veronesi U, Paganelli P, Galimberti V, Viale G, Zurrida S, Bedoni M et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative nodes. Lancet 1997; 349: 1864–1867. [DOI] [PubMed] [Google Scholar]
- 19. Weiser MR, Montgomery LL, Susnik B, Tan LK, Borgen PI, Cody HS. Is routine intraoperative frozen-section examination of sentinel lymph nodes in breast cancer worthwhile? Ann Surg Oncol 2000; 7: 651–655. [DOI] [PubMed] [Google Scholar]
- 20. Rahusen FD, Pijpers R, van Diest PJ, Bleichrodt RP, Torrenga H, Meijer S. The implementation of sentinel node biopsy as a routine procedure for patients with breast cancer. Surgery 2000; 128: 6–12. [DOI] [PubMed] [Google Scholar]
- 21. Zurrida S, Galimberti V, Orvieto E, Robertson C, Ballardini B, Cremonesi M et al. Radioguided sentinel node biopsy to avoid axillary dissection in breast cancer. Ann Surg Oncol 2000; 7: 28–31. [DOI] [PubMed] [Google Scholar]
- 22. Tanis PJ, Boom RP, Koops HS, Faneyte IF, Peterse JL, Nieweg OE et al. Frozen section investigation of the sentinel node in malignant melanoma and breast cancer. Ann Surg Oncol 2001; 8: 222–226. [DOI] [PubMed] [Google Scholar]
- 23. van de Vrande S, Meijer J, Rijnders A, Klinkenbijl JH. The value of intraoperative frozen section examination of sentinel lymph nodes in breast cancer. Eur J Surg Oncol 2009; 35: 276–280. [DOI] [PubMed] [Google Scholar]
- 24. Viale G, Bosari S, Mazzarol G, Galimberti V, Luini A, Veronesi P et al. Intraoperative examination of axillary sentinel lymph nodes in breast carcinoma patients. Cancer 1999; 85: 2433–2438. [PubMed] [Google Scholar]
- 25. Tew K, Irwig L, Matthews A, Crowe P, Macaskill P. Meta-analysis of sentinel node imprint cytology in breast cancer. Br J Surg 2005; 92: 1068–1080. [DOI] [PubMed] [Google Scholar]
- 26. Barranger E, Antoine M, Grahek D, Callard P, Uzan S. Intraoperative imprint cytology in sentinel nodes in breast cancer. J Surg Oncol 2004; 86: 128–133. [DOI] [PubMed] [Google Scholar]
- 27. Chicken DW, Kocjan G, Falzon M, Lee AC, Douek M, Sainsbury R et al. Intraoperative touch imprint cytology for the diagnosis of sentinel lymph node metastasis in breast cancer. Br J Surg 2006; 93: 572–576. [DOI] [PubMed] [Google Scholar]
- 28. Cox C, Centeno B, Dickson D, Clark J, Nicosia S, Dupont E et al. Accuracy of intraoperative imprint cytology for sentinel lymph node evaluation in the treatment of breast carcinoma. Cancer 2005; 105: 13–20. [DOI] [PubMed] [Google Scholar]
- 29. Contractor K, Gohel M, Al-Salami E, Kaur K, Aqel N, Nigar E et al. Intra-operative imprint cytology for assessing the sentinel node in breast cancer: results of its routine use over 8 years. Eur J Surg Oncol 2008; 35: 16–20. [DOI] [PubMed] [Google Scholar]
- 30. Shiver SA, Creager AJ, Geisinger K, Perrier ND, Shen P, Levine EA. Intraoperative analysis of sentinel lymph nodes by imprint cytology for cancer of the breast. Am J Surg 2002; 184: 424–427. [DOI] [PubMed] [Google Scholar]
- 31. Leikola JP, Toivonen TS, Krogerus LA, von Smitten KA, Leidenius MH. Rapid immunohistochemistry enhances the intraoperative diagnosis of sentinel lymph node metastasis in invasive lobular breast carcinoma. Cancer 2005; 104: 14–19. [DOI] [PubMed] [Google Scholar]
- 32. Salem AA, Douglas-Jones AG, Sweetland HM, Mansel RE. Intraoperative evaluation of axillary sentinel lymph nodes using touch imprint cytology and immunohistochemistry. Part II. Results. Eur J Surg Oncol 2006; 32: 484–487. [DOI] [PubMed] [Google Scholar]
- 33. Nagashima T, Suzuki M, Yagata H, Nikaido T, Horiuchi F, Koda K et al. Intraoperative cytologic diagnosis of sentinel node metastasis in breast cancer. Acta Cytol 2003; 47: 1028–1032. [DOI] [PubMed] [Google Scholar]
- 34. Leidenius MH, Krogerus LA, Toivonen TS, Von Smitten KJ. The feasibility of intraoperative diagnosis of sentinel lymph node metastasis in breast cancer. J Surg Oncol 2003; 84: 68–73. [DOI] [PubMed] [Google Scholar]
- 35. Sauer T, Engh V, Holck AM, Sørpebøl G, Heim M, Furu I et al. Imprint cytology of sentinel lymph nodes in breast cancer. Experience with rapid intraoperative diagnosis and primary screening by cytotechnologists. Acta Cytol 2003; 47: 768–773. [DOI] [PubMed] [Google Scholar]
- 36. Beach RA, Lawson D, Waldrop SM, Cohen C. Rapid immunohistochemistry for cytokeratin in the intraoperative evaluation of sentinel lymph nodes for metastatic breast carcinoma. Appl Immunohistochem Mol Morphol 2003; 11: 45–50. [DOI] [PubMed] [Google Scholar]
- 37. Cserni G. Effect of increasing the surface sampled by imprint cytology on the intraoperative assessment of axillary sentinel lymph nodes in breast cancer patients. Am Surg 2003; 69: 419–423. [PubMed] [Google Scholar]
- 38. Schoenfeld A, Laqmani Y, Smith D, O'Reilly S, Shousha S, Sinnett HD et al. Detection of breast cancer micrometastases by using polymerase chain reaction. Cancer Res 1994; 54: 2986–2990. [PubMed] [Google Scholar]
- 39. Mori M, Mimori K, Inoue H, Barnard GF, Tsuji K, Nanbara S et al. Detection of cancer micrometastasis in lymph nodes by reverse-transcriptase polymerase chain reaction. Cancer Res 1995; 55: 3417–3420. [PubMed] [Google Scholar]
- 40. Schoenfeld A, Luqmani Y, Sinnett HD, Shousha S, Coombes RC. Keratin 19 mRNA measurement to detect micrometastases in lymph nodes in breast cancer patients. Br J Cancer 1996; 76: 1639–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mori M, Koshi M, Ueo H, Karimine N, Barnard GF, Sugimachi K et al. Molecular detection of circulating solid carcinoma cells in the peripheral blood: the concept of early systemic disease. Int J Cancer 1996; 68: 739–743. [DOI] [PubMed] [Google Scholar]
- 42. Schröder CP, Ruiters MHJ, De Jong S, Tiebosch AT, Wesseling J, Veenstra R et al. Detection of micrometastatic breast cancer by means of real time quantitative RT–PCR and immunostaining in perioperative blood samples and sentinel nodes. Int J Cancer 2003; 106: 611–618. [DOI] [PubMed] [Google Scholar]
- 43. Braun S, Pantel K, Müller P, Janni W, Hepp F, Kentenich CR et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, stage II or stage III breast cancer. N Engl J Med 2000; 342: 525–533. [DOI] [PubMed] [Google Scholar]
- 44. Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC et al. Circulating tumour cells, disease progression and survival in metastatic breast cancer. N Engl J Med 2004; 351: 781–791. [DOI] [PubMed] [Google Scholar]
- 45. Gillanders WE, Mikhitarian K, Hebert R, Mauldin PD, Palesch Y, Walters C et al. Molecular detection of micrometastatic breast cancer in histopathology-negative axillary lymph nodes correlates with traditional predictors of prognosis; an interim analysis of a prospective multi-institutional cohort study. Ann Surg 2004; 239: 828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bostick PJ, Chatterjee S, Chi DD, Huynh KT, Giuliano AE, Cote R et al. Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol 1998; 16: 2632–2640. [DOI] [PubMed] [Google Scholar]
- 47. Manzotti M, Dell'Orto P, Maisonneuve P, Zurrida S, Mazzarol G, Viale G. Reverse transcription–polymerase chain reaction assay for multiple mRNA markers in the detection of breast cancer metastases in sentinel lymph nodes. Int J Cancer 2001; 95: 307–312. [DOI] [PubMed] [Google Scholar]
- 48. Sakaguchi M, Virmani A, Dudak MW, Peters GN, Leitch AM, Saboorian H et al. Clinical relevance of reverse transcriptase–polymerase chain reaction for the detection of axillary lymph node metastasis in breast cancer. Ann Surg Oncol 2003; 10: 117–125. [DOI] [PubMed] [Google Scholar]
- 49. Mitas M, Mikhitarian K, Walters C, Baron PL, Elliott BM, Brothers TE et al. Quantitative real-time RT–PCR detection of breast cancer micrometastasis using a multigene marker panel. Int J Cancer 2001; 93: 162–171. [DOI] [PubMed] [Google Scholar]
- 50. Inokuchi M, Ninomiya I, Tsugawa K, Terada I, Miwa K. Quantitative evaluation of metastases in axillary lymph nodes of breast cancer. Br J Cancer 2003; 89: 1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dell'Orto P, Biasi MO, Del Curto B, Zurrida S, Galimberti V, Viale G. Assessing the status of axillary sentinel lymph nodes of breast carcinoma patients by a real-time quantitative RT–PCR assay for mammaglobin 1 mRNA. Breast Cancer Res Treat 2006; 98: 185–190. [DOI] [PubMed] [Google Scholar]
- 52. Backus J, Laughlin T, Wang Y, Belly R, White R, Baden J et al. Identification and characterization of optimal gene expression markers for detection of breast cancer metastasis. J Mol Diagn 2005; 7: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hughes SJ, Liqiang X, Raja S, Gooding W, Cole DJ, Gillanders WE et al. A rapid, fully automated, molecular-based assay accurately analyses sentinel lymph nodes for the presence of metastatic breast cancer. Ann Surg 2006; 243: 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Loftus P. Doctors thought discontinued J&J cancer test was impractical. http://online.wsj.com/article/BT-CO-20100125-710277.html. The Wall Street Journal 25 January 2010.
- 55. Julian TB, Blumencranz P, Deck K, Whitworth P, Berry DA, Berry SM et al. Novel intraoperative molecular test for sentinel lymph node metastasis in patients with early-stage breast cancer. J Clin Oncol 2008; 26: 3338–3345. [DOI] [PubMed] [Google Scholar]
- 56. Blumencranz P, Whitworth PW, Deck K, Rosenberg A, Reintgen D, Beitsch P et al. Scientific Impact Recognition Award. Sentinel node staging for breast cancer: intraoperative molecular pathology overcomes conventional histologic sampling errors. Am J Surg 2007; 194: 426–432. [DOI] [PubMed] [Google Scholar]
- 57. Viale G, Dell'Orto P, Biasi MO, Stufano V, De Brito Lima LN, Paganelli G et al. Comparative evaluation of an extensive histopathologic examination and a real-time reverse-transcription–polymerase chain reaction assay for mammaglobin and cytokeratin 19 on axillary sentinel lymph nodes of breast carcinoma patients. Ann Surg 2008; 247: 136–142. [DOI] [PubMed] [Google Scholar]
- 58. Martinez M, Veys I, Majjaj S, Lespagnard L, Schobbens JC, Rouas G et al. Clinical validation of a molecular assay for intra-operative detection of metastases in breast sentinel lymph nodes. Eur J Surg Oncol 2009; 35: 387–392. [DOI] [PubMed] [Google Scholar]
- 59. Mansel RE, Goyal A, Douglas-Jones A, Woods V, Goyal S, Monypenny I et al. Detection of breast cancer metastasis in sentinel lymph nodes using intra-operative real time GeneSearch BLN assay in the operating room: results of the Cardiff study. Breast Cancer Res Treat 2009; 115: 595–600. [DOI] [PubMed] [Google Scholar]
- 60. Veys I, Majjaj S, Salgado R, Noterman D, Schobbens JC, Manouach F et al. Evaluation of the histological size of the sentinel lymph node metastases using RT–PCR assay: a rapid tool to estimate the risk of non-sentinel lymph node invasion in patients with breast cancer. Breast Cancer Res Treat 2009; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 61. Tafe LJ, Schwab MC, Lefferts JA, Wells WA, Tsongalis GJ. A validation study of a new molecular diagnostic assay: the Dartmouth-Hitchcock Medical Centre experience with the GeneSearch BLN assay in breast sentinel lymph nodes. Exp Mol Pathol 2010; 88: 1–6. [DOI] [PubMed] [Google Scholar]
- 62. Cutress RI, McDowell A, Gabriel FG, Gill J, Jeffery MJ, Agrawal A et al. Observational and cost analysis of the implementation of breast cancer sentinel node intra-operative molecular diagnosis. J Clin Pathol 2010; 6: 522–529. [DOI] [PubMed] [Google Scholar]
- 63. Eiken Genome Site . The Principles of LAMP Method. 2005; http://loopamp.eiken.co.jp/e/lamp/index.html [accessed 23 November 2009]. [Google Scholar]
- 64. Visser M, Jiwa M, Horstman A, Brink AA, Pol RP, van Diest P et al. Intra-operative rapid diagnostic method based on CK19 mRNA expression for the detection of lymph node metastasis in breast cancer. Int J Cancer 2008; 122: 2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tsujimoto M, Nakabayashi K, Yoshidome K, Kaneka T, Iwase T, Akiyama F et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res 2007; 13: 4807–4816. [DOI] [PubMed] [Google Scholar]
- 66. Schem C, Maass N, Bouerschlag DO, Carstensen MH, Löning T, Roder C et al. One-step nucleic acid amplification—a molecular method for the detection of lymph node metastases in breast cancer patients; results of the German study group. Virchows Arch 2009; 454: 203–210. [DOI] [PubMed] [Google Scholar]
- 67. Lovat LB, Johnson K, Mackenzie GD, Clark BR, Novelli MR, Davies S et al. Elastic scattering spectroscopy accurately detects high grade dysplasia and cancer in Barrett's oesophagus. Gut 2006; 55: 1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Johnson KS, Chicken DW, Pickard DC, Lee AC, Briggs G, Falzon M et al. Elastic scattering spectroscopy for intraoperative determination of lymph node status in the breast. J Biomed Opt 2004; 9: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 69. Veronesi U, Zurrida S, Mazzarol G, Viale G. Extensive frozen section examination of axillary sentinel nodes to determine selective axillary dissection. World J Surg 2001; 25: 806–808. [DOI] [PubMed] [Google Scholar]
- 70. Weigelt B, Verduijn P, Bosma AJ, Rutgers EJ, Peterse HL, van't Veer LJ. Detection of metastases in sentinel lymph nodes of breast cancer patients by multiple mRNA markers. Br J Cancer 2004; 90: 1531–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yiangou C, Shousha S, Sinnett HD. Primary tumour characteristics and axillary lymph node status in breast cancer. Br J Cancer 1999; 80: 1974–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Roberts CA, Beitsch PD, Litz CE, Hilton DS, Ewing GE, Clifford E et al. Interpretive disparity among pathologists in breast sentinel lymph node evaluation. Am J Surg 2003; 186: 324–329. [DOI] [PubMed] [Google Scholar]
- 73. Rutledge H, Davis J, Chiu R, Cibull M, Brill Y, McGrath P et al. Sentinel node micrometastasis in breast carcinoma may not be an indication for complete axillary dissection. Mod Pathol 2005; 18: 762–768. [DOI] [PubMed] [Google Scholar]
- 74. Cserni G, Gregori D, Merletti F, Sapino A, Mano MP, Ponti A et al. Meta-analysis of non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer. Br J Surg 2004; 91: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 75. Prognostic importance of occult axillary lymph node micrometastases from breast cancers . International (Ludwig) Breast Cancer Study Group. Lancet 1990; 335: 1565–1568. [PubMed] [Google Scholar]
- 76. Clare SE, Sener SF, Wilkens W, Goldschmidt R, Merkel D, Winchester DJ. Prognostic significance of occult lymph node metastases in node-negative breast cancer. Ann Surg Oncol 1997; 4: 447–451. [DOI] [PubMed] [Google Scholar]
- 77. Rutgers EJT. Sentinel node biopsy: interpretation and management of patients with immunohistochemistry-positive sentinel nodes and those with micrometastases. J Clin Oncol 2008; 26: 698–702. [DOI] [PubMed] [Google Scholar]
- 78. Keshtgar MRS, Chicken DW, Austwick MR, Somasundaram SK, Mosse CA, Zhu Y et al. Optical scanning for rapid intraoperative diagnosis of sentinel node metastases in breast cancer. Br J Surg 2010; 97: 1232–1239. [DOI] [PubMed] [Google Scholar]
- 79. Tamaki Y, Akiyama F, Iwase T, Kaneko T, Tsuda H, Sato K et al. Molecular detection of lymph node metastases in breast cancer patients: results of a multicenter trial using the one-step nucleic acid amplification assay. Clin Cancer Res 2009; 15: 2879–2884. [DOI] [PubMed] [Google Scholar]


