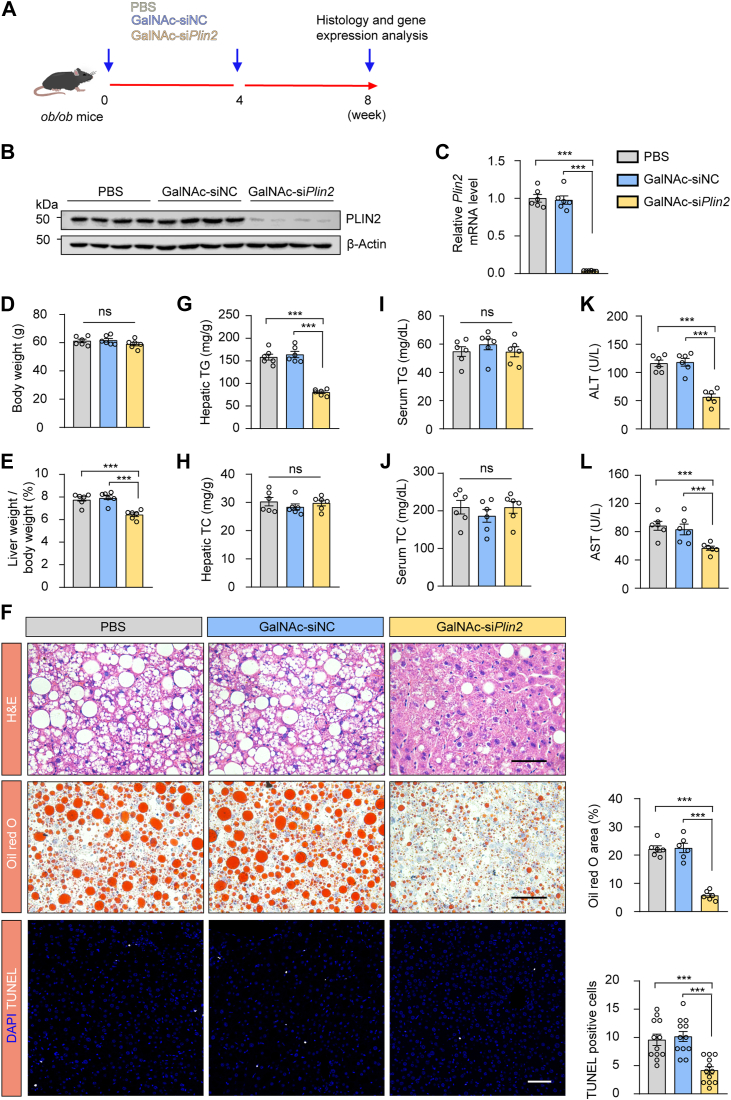
Fig. 4.
Treatment with GalNAc-siPlin2 ameliorates MASLD induced by obesity. A; Schematic representation of obesity-induced MASLD in leptin-deficient (ob/ob) mice subcutaneously injected with 4 mg/kg GalNAc-siPlin2, GalNAc-siNC, or PBS. B: RT-qPCR analysis of relative mRNA levels of hepatic Plin2 in ob/ob mice subcutaneously injected with 4 mg/kg GalNAc-siPlin2, GalNAc-siNC, or PBS. C: Immunoblotting analysis of hepatic PLIN2 protein levels in ob/ob mice subcutaneously injected with 4 mg/kg GalNAc-siPlin2, GalNAc-siNC, or PBS. D,E: Body weight (D) and liver-to-body weight ratio (E) for ob/ob mice subcutaneously injected with 4 mg/kg GalNAc-siPlin2, GalNAc-siNC, or PBS. F: Histological analysis of liver sections from ob/ob mice subcutaneously injected with 4 mg/kg GalNAc-siPlin2, GalNAc-siNC, or PBS. Quantification of Oil red O staining–positive areas and TUNEL-positive cells is shown. Scale bar, 50 μm. G-J: The levels of hepatic TG (G), hepatic TC (H), serum TG (I), and serum TC (J) from ob/ob mice subcutaneously injected with 4 mg/kg GalNAc-siPlin2, GalNAc-siNC, or PBS. K,L: The levels of serum ALT (K) and serum AST (L) in ob/ob mice subcutaneously injected with 4 mg/kg GalNAc-siPlin2, GalNAc-siNC, or PBS. Data are shown as mean ± SEM. For all the panels, n = 6 mice per group. P values were calculated using one-way ANOVA followed by Tukey's multiple comparison tests. “ns” denotes no significant difference. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. ALT, alanine aminotransferase; AST, aspartate aminotransferase; GalNAc, N-acetylgalactosamine; MASLD, metabolic dysfunction–associated steatotic liver disease; RT-qPCR, quantitative real-time PCR; TC, total cholesterol; TG, triglyceride.